Targeting the IL-6-Yap-Snail signalling axis in synovial fibroblasts ameliorates inflammatory arthritis
- PMID: 34844926
- PMCID: PMC8762018
- DOI: 10.1136/annrheumdis-2021-220875
Targeting the IL-6-Yap-Snail signalling axis in synovial fibroblasts ameliorates inflammatory arthritis
Abstract
Objective: We aimed to understand the role of the transcriptional co-factor Yes-associated protein (Yap) in the molecular pathway underpinning the pathogenic transformation of synovial fibroblasts (SF) in rheumatoid arthritis (RA) to become invasive and cause joint destruction.
Methods: Synovium from patients with RA and mice with antigen-induced arthritis (AIA) was analysed by immunostaining and qRT-PCR. SF were targeted using Pdgfrα-CreER and Gdf5-Cre mice, crossed with fluorescent reporters for cell tracing and Yap-flox mice for conditional Yap ablation. Fibroblast phenotypes were analysed by flow cytometry, and arthritis severity was assessed by histology. Yap activation was detected using Yap-Tead reporter cells and Yap-Snail interaction by proximity ligation assay. SF invasiveness was analysed using matrigel-coated transwells.
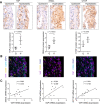
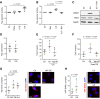
Results: Yap, its binding partner Snail and downstream target connective tissue growth factor were upregulated in hyperplastic human RA and in mouse AIA synovium, with Yap detected in SF but not macrophages. Lineage tracing showed polyclonal expansion of Pdgfrα-expressing SF during AIA, with predominant expansion of the Gdf5-lineage SF subpopulation descending from the embryonic joint interzone. Gdf5-lineage SF showed increased expression of Yap and adopted an erosive phenotype (podoplanin+Thy-1 cell surface antigen-), invading cartilage and bone. Conditional ablation of Yap in Gdf5-lineage cells or Pdgfrα-expressing fibroblasts ameliorated AIA. Interleukin (IL)-6, but not tumour necrosis factor alpha (TNF-α) or IL-1β, Jak-dependently activated Yap and induced Yap-Snail interaction. SF invasiveness induced by IL-6 stimulation or Snail overexpression was prevented by Yap knockdown, showing a critical role for Yap in SF transformation in RA.
Conclusions: Our findings uncover the IL-6-Yap-Snail signalling axis in pathogenic SF in inflammatory arthritis.
Keywords: arthritis; experimental; fibroblasts; inflammation; rheumatoid.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

