Timing of dialysis initiation to reduce mortality and cardiovascular events in advanced chronic kidney disease: nationwide cohort study
- PMID: 34844936
- PMCID: PMC8628190
- DOI: 10.1136/bmj-2021-066306
Timing of dialysis initiation to reduce mortality and cardiovascular events in advanced chronic kidney disease: nationwide cohort study
Abstract
Objective: To identify the optimal estimated glomerular filtration rate (eGFR) at which to initiate dialysis in people with advanced chronic kidney disease.
Design: Nationwide observational cohort study.
Setting: National Swedish Renal Registry of patients referred to nephrologists.
Participants: Patients had a baseline eGFR between 10 and 20 mL/min/1.73 m2 and were included between 1 January 2007 and 31 December 2016, with follow-up until 1 June 2017.
Main outcome measures: The strict design criteria of a clinical trial were mimicked by using the cloning, censoring, and weighting method to eliminate immortal time bias, lead time bias, and survivor bias. A dynamic marginal structural model was used to estimate adjusted hazard ratios and absolute risks for five year all cause mortality and major adverse cardiovascular events (composite of cardiovascular death, non-fatal myocardial infarction, or non-fatal stroke) for 15 dialysis initiation strategies with eGFR values between 4 and 19 mL/min/1.73 m2 in increments of 1 mL/min/1.73 m2. An eGFR between 6 and 7 mL/min/1.73 m2 (eGFR6-7) was taken as the reference.
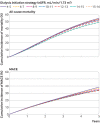
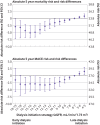
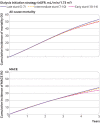
Results: Among 10 290 incident patients with advanced chronic kidney disease (median age 73 years; 3739 (36%) women; median eGFR 16.8 mL/min/1.73 m2), 3822 started dialysis, 4160 died, and 2446 had a major adverse cardiovascular event. A parabolic relation was observed for mortality, with the lowest risk for eGFR15-16. Compared with dialysis initiation at eGFR6-7, initiation at eGFR15-16 was associated with a 5.1% (95% confidence interval 2.5% to 6.9%) lower absolute five year mortality risk and 2.9% (0.2% to 5.5%) lower risk of a major adverse cardiovascular event, corresponding to hazard ratios of 0.89 (95% confidence interval 0.87 to 0.92) and 0.94 (0.91 to 0.98), respectively. This 5.1% absolute risk difference corresponded to a mean postponement of death of 1.6 months over five years of follow-up. However, dialysis would need to be started four years earlier. When emulating the intended strategies of the Initiating Dialysis Early and Late (IDEAL) trial (eGFR10-14 v eGFR5-7) and the achieved eGFRs in IDEAL (eGFR7-10 v eGFR5-7), hazard ratios for all cause mortality were 0.96 (0.94 to 0.99) and 0.97 (0.94 to 1.00), respectively, which are congruent with the findings of the randomised IDEAL trial.
Conclusions: Very early initiation of dialysis was associated with a modest reduction in mortality and cardiovascular events. For most patients, such a reduction may not outweigh the burden of a substantially longer period spent on dialysis.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: support from the Swedish Research Council, Center of Innovative Medicine Karolinska, and Stockholm City Council. JJC has consulted for Baxter and AstraZeneca and received grant support from AstraZeneca, Viforpharma, and Astellas, all outside the submitted work; CMC has received consultation, advisory board membership, or research funding from the Ontario Ministry of Health, Sanofi, Johnson and Johnson, Pfizer, Leo Pharma, Astellas, Janssen, Amgen, Boehringer-Ingelheim, and Baxter, all outside the submitted work; CW has received consultation and advisory board membership honorariums from AstraZeneca, Akebia, Bayer, Boehringer-Ingelheim, Gilead, GSK, MSD, Sanofi-Genzyme, and Tricida, outside the present work. KJJ has received grant support from the European Renal Association-European Dialysis and Transplant Association (ERA-EDTA) and speaker honorariums from Fresenius Medical Care, outside the submitted work. FJC has received grant support from the National Institute for Health Research and Kidney Research UK and honorariums from Baxter. ME has received payment for advisory boards and lectures and research funding from Astellas Pharma, Astra Zeneca, Vifor Pharma, and Fresenius, all outside the submitted work; no other relationships or activities that could appear to have influenced the submitted work.
Figures

References
-
- United States Renal Data System. 2020 USRDS Annual Data Report: Epidemiology of kidney disease in the United States. 2020. https://adr.usrds.org/2020/. - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
