Rapid Assay for Sick Children with Acute Lung infection Study (RASCALS): diagnostic cohort study protocol
- PMID: 34845080
- PMCID: PMC8634010
- DOI: 10.1136/bmjopen-2021-056197
Rapid Assay for Sick Children with Acute Lung infection Study (RASCALS): diagnostic cohort study protocol
Abstract
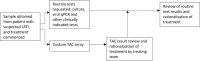
Introduction: Lower respiratory tract infection (LRTI) is the most commonly treated infection in critically ill children. Pathogens are infrequently identified on routine respiratory culture, and this is a time-consuming process. A syndromic approach to rapid molecular testing that includes a wide range of bacterial and fungal targets has the potential to aid clinical decision making and reduce unnecessary broad spectrum antimicrobial prescribing. Here, we describe a single-centre prospective cohort study investigating the use of a 52-pathogen TaqMan array card (TAC) for LRTI in the paediatric intensive care unit (PICU).
Methods and analysis: Critically ill children with suspected LRTI will be enrolled to this 100 patient single-centre prospective observational study in a PICU in the East of England. Samples will be obtained via routine non-bronchoscopic bronchoalveolar lavage which will be sent for standard microbiology culture in addition to TAC. A blood draw will be obtained via any existing vascular access device. The primary outcomes of the study will be (1) concordance of TAC result with routine culture and 16S rRNA gene sequencing and (2) time of diagnostic result from TAC versus routine culture. Secondary outcomes will include impact of the test on total antimicrobial prescriptions, a description of the inflammatory profile of the lung and blood in response to pneumonia and a description of the clinical experience of medical and nursing staff using TAC.
Ethics and dissemination: This study has been approved by the Yorkshire and the Humber-Bradford Leeds Research Ethics Committee (REC reference 20/YH/0089). Informed consent will be obtained from all participants. Results will be published in peer-reviewed publications and international conferences.
Trial registration number: NCT04233268.
Keywords: molecular diagnostics; paediatric intensive & critical care; respiratory infections.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: MDC is the inventor on a patent held by the Secretary of State for Health (UK government) EP2788503, which covers some of the genetic sequences used in this study. VN is a founder, director and shareholder in Cambridge Infection Diagnostics (CID) which is a commercial company aimed at developing molecular diagnostics in infection and antimicrobial and AMR stewardship. ACM, SB and ED are members of the Scientific Advisory Board of Cambridge Infection Diagnostics (CID). TG has received a research grant from Shionogi. All other authors declare no conflict of interest.
Figures
References
-
- Paediatric Intensive Care Audit Network . Paediatric Intensive Care Audit Network Annual Report 2019; 2019.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical