Chemotoxicity-induced exosomal lncFERO regulates ferroptosis and stemness in gastric cancer stem cells
- PMID: 34845198
- PMCID: PMC8629982
- DOI: 10.1038/s41419-021-04406-z
Chemotoxicity-induced exosomal lncFERO regulates ferroptosis and stemness in gastric cancer stem cells
Abstract
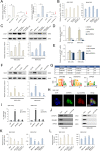
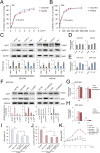
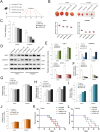
Cancer stem cells (CSCs) are an important cause of tumor recurrence and drug resistance. As a new type of cell death that relies on iron ions and is strictly regulated by intracellular and extracellular signals, the role of ferroptosis in tumor stem cells deserves extensive attention. Mass spectrum was applied to screen for ferroptosis-related proteins in gastric cancer (GC). Sphere-formation assay was used to estimate the stemness of gastric cancer stem cells (GCSCs). Exosomal lnc-ENDOG-1:1 (lncFERO) was isolated by ultracentrifugation. Ferroptosis was induced by erastin and was assessed by detecting lipid ROS, mitochondrial membrane potential, and cell death. Furthermore, a series of functional in vitro and in vivo experiments were conducted to evaluate the effects of lncFERO on regulating ferroptosis and chemosensitivity in GCSCs. Here, we showed that stearoyl-CoA-desaturase (SCD1) played a key role in regulating lipid metabolism and ferroptosis in GCSCs. Importantly, exosomal lncFERO (exo-lncFERO) derived from GC cells was demonstrated to promote SCD1 expression by directly interacting with SCD1 mRNA and recruiting heterogeneous nuclear ribonucleoprotein A1 (hnRNPA1), which resulted in the dysregulation of PUFA levels and the suppression of ferroptosis in GCSCs. Moreover, we found that hnRNPA1 was also involved in lncFERO packing into exosomes in GC cells, and both in vitro and in vivo data suggested that chemotoxicity induced lncFERO secretion from GC cells by upregulating hnRNPA1 expression, leading to enhanced stemness and acquired chemo-resistance. All these data suggest that GC cells derived exo-lncFERO controls GCSC tumorigenic properties through suppressing ferroptosis, and targeting exo-lncFERO/hnRNPA1/SCD1 axis combined with chemotherapy could be a promising CSC-based strategy for the treatment of GC.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
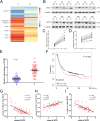
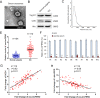
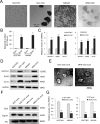
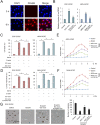

References
-
- Giraud J, Molina-Castro S, Seeneevassen L, Sifré E, Izotte J, Tiffon C, et al. Verteporfin targeting YAP1/TAZ-TEAD transcriptional activity inhibits the tumorigenic properties of gastric cancer stem cells. Int J Cancer. 2020;146:2255–67. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

