Angiogenin mediates paternal inflammation-induced metabolic disorders in offspring through sperm tsRNAs
- PMID: 34845238
- PMCID: PMC8630171
- DOI: 10.1038/s41467-021-26909-1
Angiogenin mediates paternal inflammation-induced metabolic disorders in offspring through sperm tsRNAs
Abstract
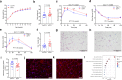
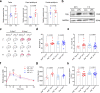
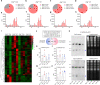
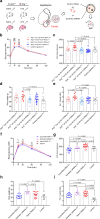
Paternal environmental inputs can influence various phenotypes in offspring, presenting tremendous implications for basic biology and public health and policy. However, which signals function as a nexus to transmit paternal environmental inputs to offspring remains unclear. Here we show that offspring of fathers with inflammation exhibit metabolic disorders including glucose intolerance and obesity. Deletion of a mouse tRNA RNase, Angiogenin (Ang), abolished paternal inflammation-induced metabolic disorders in offspring. Additionally, Ang deletion prevented the inflammation-induced alteration of 5'-tRNA-derived small RNAs (5'-tsRNAs) expression profile in sperm, which might be essential in composing a sperm RNA 'coding signature' that is needed for paternal epigenetic memory. Microinjection of sperm 30-40 nt RNA fractions (predominantly 5'-tsRNAs) from inflammatory Ang+/+ males but not Ang-/- males resulted in metabolic disorders in the resultant offspring. Moreover, zygotic injection with synthetic 5'-tsRNAs which increased in inflammatory mouse sperm and decreased by Ang deletion partially resembled paternal inflammation-induced metabolic disorders in offspring. Together, our findings demonstrate that Ang-mediated biogenesis of 5'-tsRNAs in sperm contributes to paternal inflammation-induced metabolic disorders in offspring.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interest.
Figures
References
-
- Chen Q, et al. Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder. Science. 2016;351:397–400. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

