Photocrosslinked gelatin hydrogel improves wound healing and skin flap survival by the sustained release of basic fibroblast growth factor
- PMID: 34845307
- PMCID: PMC8630120
- DOI: 10.1038/s41598-021-02589-1
Photocrosslinked gelatin hydrogel improves wound healing and skin flap survival by the sustained release of basic fibroblast growth factor
Abstract
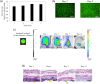
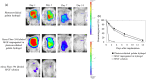
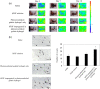
Biomaterials traditionally used for wound healing can act as a temporary barrier to halt bleeding, prevent infection, and enhance regeneration. Hydrogels are among the best candidates for wound healing owing to their moisture retention and drug-releasing properties. Photo-polymerization using visible light irradiation is a promising method for hydrogel preparation since it can easily control spatiotemporal reaction kinetics and rapidly induce a single-step reaction under mild conditions. In this study, photocrosslinked gelatin hydrogels were imparted with properties namely fast wound adherence, strong wet tissue surface adhesion, greater biocompatibility, long-term bFGF release, and importantly, ease of use through the modification and combination of natural bio-macromolecules. The production of a gelatin hydrogel made of natural gelatin (which is superior to chemically modified gelatin), crosslinked by visible light, which is more desirable than UV light irradiation, will enable its prolonged application to uneven wound surfaces. This is due to its flexible shape, along with the administration of cell growth factors, such as bFGF, for tissue regeneration. Further, the sustained release of bFGF enhances wound healing and skin flap survival. The photocrosslinking gelatin hydrogel designed in this study is a potential candidate to enhance wound healing and better skin flap survival.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

