The implications of hyperoxia, type 1 diabetes and sex on cardiovascular physiology in mice
- PMID: 34845324
- PMCID: PMC8630164
- DOI: 10.1038/s41598-021-02550-2
The implications of hyperoxia, type 1 diabetes and sex on cardiovascular physiology in mice
Abstract
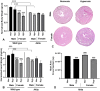
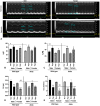
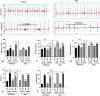
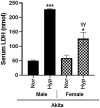
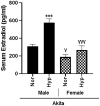
Oxygen supplementation, although a cornerstone of emergency and cardiovascular medicine, often results in hyperoxia, a condition characterized by excessive tissue oxygen which results in adverse cardiac remodeling and subsequent injurious effects to physiological function. Cardiac remodeling is further influenced by various risk factors, including pre-existing conditions and sex. Thus, the purpose of this experiment was to investigate cardiac remodeling in Type I Diabetic (Akita) mice subjected to hyperoxic treatment. Overall, we demonstrated that Akita mice experience distinct challenges from wild type (WT) mice. Specifically, Akita males at both normoxia and hyperoxia showed significant decreases in body and heart weights, prolonged PR, QRS, and QTc intervals, and reduced %EF and %FS at normoxia compared to WT controls. Moreover, Akita males largely resemble female mice (both WT and Akita) with regards to the parameters studied. Finally, statistical analysis revealed hyperoxia to have the greatest influence on cardiac pathophysiology, followed by sex, and finally genotype. Taken together, our data suggest that Type I diabetic patients may have distinct cardiac pathophysiology under hyperoxia compared to uncomplicated patients, with males being at high risk. These findings can be used to enhance provision of care in ICU patients with Type I diabetes as a comorbid condition.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

