Serine catabolism generates liver NADPH and supports hepatic lipogenesis
- PMID: 34845393
- PMCID: PMC8721747
- DOI: 10.1038/s42255-021-00487-4
Serine catabolism generates liver NADPH and supports hepatic lipogenesis
Abstract
Carbohydrate can be converted into fat by de novo lipogenesis, a process upregulated in fatty liver disease. Chemically, de novo lipogenesis involves polymerization and reduction of acetyl-CoA, using NADPH as the electron donor. The feedstocks used to generate acetyl-CoA and NADPH in lipogenic tissues remain, however, unclear. Here we show using stable isotope tracing in mice that de novo lipogenesis in adipose is supported by glucose and its catabolism via the pentose phosphate pathway to make NADPH. The liver, in contrast, derives acetyl-CoA for lipogenesis from acetate and lactate, and NADPH from folate-mediated serine catabolism. Such NADPH generation involves the cytosolic serine pathway in liver running in the opposite direction to that observed in most tissues and tumours, with NADPH made by the SHMT1-MTHFD1-ALDH1L1 reaction sequence. SHMT inhibition decreases hepatic lipogenesis. Thus, liver folate metabolism is distinctively wired to support cytosolic NADPH production and lipogenesis. More generally, while the same enzymes are involved in fat synthesis in liver and adipose, different substrates are used, opening the door to tissue-specific pharmacological interventions.
© 2021. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Competing Interests Statement
G.X., G.J.T. and M.F.C. are current employees of Pfizer and may be Pfizer shareholders. J.D.R. is a consultant and received research funding from Pfizer and is an advisor and stock owner in Colorado Research Partners, L.E.A.F. Pharmaceuticals, Rafael Pharmaceuticals, Barer Institute and its subsidiaries, Serien Therapeutics, Toran, Kadmon Pharmaceuticals, and Agios Pharmaceuticals. J.D.R. is co-inventor of SHIN2 and related SHMT inhibitors, which have been patented by Princeton University. The remaining authors declare no competing interests.
Figures









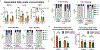


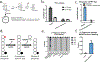

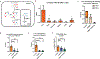
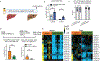
Comment in
-
Revisiting the role of serine metabolism in hepatic lipogenesis.Nat Metab. 2023 May;5(5):760-761. doi: 10.1038/s42255-023-00792-0. Epub 2023 May 11. Nat Metab. 2023. PMID: 37169876 No abstract available.
-
Reply to: revisiting the role of serine metabolism in hepatic lipogenesis.Nat Metab. 2023 May;5(5):762-764. doi: 10.1038/s42255-023-00793-z. Epub 2023 May 11. Nat Metab. 2023. PMID: 37169877 No abstract available.
References
-
- Lehninger AL, Nelson DL, Cox MM. Lehninger principles of biochemistry 6th ed. W.H. Freeman; 2013.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

