Probing Affinity, Avidity, Anticooperativity, and Competition in Antibody and Receptor Binding to the SARS-CoV-2 Spike by Single Particle Mass Analyses
- PMID: 34845440
- PMCID: PMC8577368
- DOI: 10.1021/acscentsci.1c00804
Probing Affinity, Avidity, Anticooperativity, and Competition in Antibody and Receptor Binding to the SARS-CoV-2 Spike by Single Particle Mass Analyses
Abstract
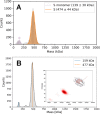
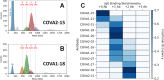
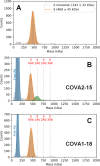
Determining how antibodies interact with the spike (S) protein of the SARS-CoV-2 virus is critical for combating COVID-19. Structural studies typically employ simplified, truncated constructs that may not fully recapitulate the behavior of the original complexes. Here, we combine two single particle mass analysis techniques (mass photometry and charge-detection mass spectrometry) to enable the measurement of full IgG binding to the trimeric SARS-CoV-2 S ectodomain. Our experiments reveal that antibodies targeting the S-trimer typically prefer stoichiometries lower than the symmetry-predicted 3:1 binding. We determine that this behavior arises from the interplay of steric clashes and avidity effects that are not reflected in common antibody constructs (i.e., Fabs). Surprisingly, these substoichiometric complexes are fully effective at blocking ACE2 binding despite containing free receptor binding sites. Our results highlight the importance of studying antibody/antigen interactions using complete, multimeric constructs and showcase the utility of single particle mass analyses in unraveling these complex interactions.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare the following competing financial interest(s): Amsterdam UMC filed a patent application on SARS-CoV-2 monoclonal antibodies including the ones used in this manuscript.
Figures



References
-
- Zhu N.; Zhang D.; Wang W.; Li X.; Yang B.; Song J.; Zhao X.; Huang B.; Shi W.; Lu R.; Niu P.; Zhan F.; Ma X.; Wang D.; Xu W.; Wu G.; Gao G. F.; Tan W. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382 (8), 727–733. 10.1056/NEJMoa2001017. - DOI - PMC - PubMed
-
- Zhou P.; Yang X.-L.; Wang X.-G.; Hu B.; Zhang L.; Zhang W.; Si H.-R.; Zhu Y.; Li B.; Huang C.-L.; Chen H.-D.; Chen J.; Luo Y.; Guo H.; Jiang R.-D.; Liu M.-Q.; Chen Y.; Shen X.-R.; Wang X.; Zheng X.-S.; Zhao K.; Chen Q.-J.; Deng F.; Liu L.-L.; Yan B.; Zhan F.-X.; Wang Y.-Y.; Xiao G.-F.; Shi Z.-L. A Pneumonia Outbreak Associated with a New Coronavirus of Probable Bat Origin. Nature 2020, 579 (7798), 270–273. 10.1038/s41586-020-2012-7. - DOI - PMC - PubMed
-
- Hoffmann M.; Kleine-Weber H.; Schroeder S.; Krüger N.; Herrler T.; Erichsen S.; Schiergens T. S.; Herrler G.; Wu N.-H.; Nitsche A.; Müller M. A.; Drosten C.; Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181 (2), 271–280. 10.1016/j.cell.2020.02.052. - DOI - PMC - PubMed
-
- Corbett K. S.; Edwards D. K.; Leist S. R.; Abiona O. M.; Boyoglu-Barnum S.; Gillespie R. A.; Himansu S.; Schäfer A.; Ziwawo C. T.; DiPiazza A. T.; Dinnon K. H.; Elbashir S. M.; Shaw C. A.; Woods A.; Fritch E. J.; Martinez D. R.; Bock K. W.; Minai M.; Nagata B. M.; Hutchinson G. B.; Wu K.; Henry C.; Bahl K.; Garcia-Dominguez D.; Ma L.; Renzi I.; Kong W.-P.; Schmidt S. D.; Wang L.; Zhang Y.; Phung E.; Chang L. A.; Loomis R. J.; Altaras N. E.; Narayanan E.; Metkar M.; Presnyak V.; Liu C.; Louder M. K.; Shi W.; Leung K.; Yang E. S.; West A.; Gully K. L.; Stevens L. J.; Wang N.; Wrapp D.; Doria-Rose N. A.; Stewart-Jones G.; Bennett H.; Alvarado G. S.; Nason M. C.; Ruckwardt T. J.; McLellan J. S.; Denison M. R.; Chappell J. D.; Moore I. N.; Morabito K. M.; Mascola J. R.; Baric R. S.; Carfi A.; Graham B. S. SARS-CoV-2 MRNA Vaccine Design Enabled by Prototype Pathogen Preparedness. Nature 2020, 586 (7830), 567–571. 10.1038/s41586-020-2622-0. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

