Comparison of Antibody Response Elicited by ChAdOx1 and BNT162b2 COVID-19 Vaccine
- PMID: 34845875
- PMCID: PMC8629719
- DOI: 10.3346/jkms.2021.36.e311
Comparison of Antibody Response Elicited by ChAdOx1 and BNT162b2 COVID-19 Vaccine
Abstract
Background: ChAdOx1 and BNT162b2 vaccines are currently commonly used against coronavirus disease 2019 worldwide. Our study was designed to determine the serostatus and relative levels of anti-S and neutralizing antibodies in patients who were administered either ChAdOx1 or BNT162b2 vaccine. In addition, we investigated whether the antibody response to each vaccine differed according to sex and age.
Methods: Healthcare workers (HCWs) at a general hospital who were vaccinated with two doses of either ChAdOx1 or BNT162b2 were invited to participate in this prospective cohort study. Blood samples of HCWs vaccinated with both ChAdOx1 doses over a period of 12 weeks were collected at weeks 4 and 8 post first vaccination and 2 weeks post second vaccination. Blood samples of HCWs vaccinated with BNT162b2 were collected in the third week after the first dose, and the second dose was then administered on the same day; two weeks post second dose (5 weeks after the first dose), blood samples were collected to assess the antibody response. The titers of anti-S antibodies against the severe acute respiratory syndrome coronavirus 2 spike (S) protein receptor-binding domain and the neutralizing antibodies in the collected blood were evaluated.
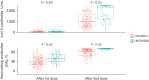
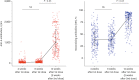
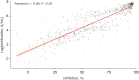
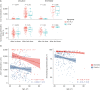
Results: Of the 309 HCWs enrolled in the study, 205 received ChAdOx1 and 104 received BNT162b2. Blood samples from participants receiving either the ChAdOx1 or BNT162b2 vaccine exhibited substantial anti-S and neutralizing antibody seropositivity subsequent to the second dose. All participants (100%) from both vaccine groups were seropositive for anti-S antibody, while 98% (201/205) of ChAdOx1-vaccinated individuals and 100% (104/104) of BNT162b2-vaccinated individuals were seropositive for neutralizing antibodies. The median levels of anti-S and neutralizing antibodies were significantly higher in the BNT162b2-vaccinated group than the ChAdOx1-vaccinated group; in particular, anti-S antibody titers of 1,020 (interquartile range, 571.0-1,631.0) U/mL vs. 2,360 (1,243-2,500) U/mL, P < 0.05, were recorded for the ChAdOx1 and BNT162b2 groups, respectively, and neutralizing antibody titers of 85.0 (65.9-92.1%) vs. 95.8 (94.4-96.6%), P < 0.05, were recorded for the ChAdOx1 and BNT162b2 groups, respectively. In the ChAdOx1 vaccine group, the neutralizing antibody level was significantly higher in women than in men (85.7 [70.3-92.5%] vs. 77.7 [59.2-91.0%], P < 0.05); however, the neutralizing antibody titer in the BNT162b2 vaccine group did not vary between the two sexes (95.9 [95.2-96.6%] vs. 95.2 [93.5-96.3%], P = 0.200). Analysis of the correlation of antibody profiles with age revealed that the levels of anti-S antibodies and signal inhibition rate (SIR) of neutralizing antibodies decreased significantly with age.
Conclusion: Both the ChAdOx1- and BNT162b2-vaccinated groups showed high seropositivity for anti-S and neutralizing antibodies. The SIR of neutralizing antibodies in the ChAdOx1 vaccine group was higher in women than in men. Enhanced antibody responses were observed in participants vaccinated with BNT162b2 compared to those vaccinated with the ChAdOx1 vaccine.
Keywords: Antibody; COVID-19 Vaccine; Immunogenicity; SARS-CoV-2.
© 2021 The Korean Academy of Medical Sciences.
Conflict of interest statement
The authors have no potential conflicts of interest to disclose.
Figures

References
-
- Ramasamy MN, Minassian AM, Ewer KJ, Flaxman AL, Folegatti PM, Owens DR, et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2021;396(10267):1979–1993. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

