New Insights into the Structure-Activity Relationship and Neuroprotective Profile of Benzodiazepinone Derivatives of Neurounina-1 as Modulators of the Na+/Ca2+ Exchanger Isoforms
- PMID: 34845907
- PMCID: PMC8713167
- DOI: 10.1021/acs.jmedchem.1c01212
New Insights into the Structure-Activity Relationship and Neuroprotective Profile of Benzodiazepinone Derivatives of Neurounina-1 as Modulators of the Na+/Ca2+ Exchanger Isoforms
Abstract
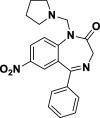
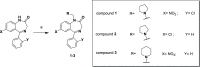
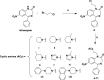
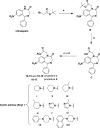
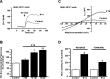
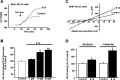
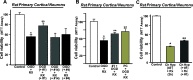
Due to the neuroprotective role of the Na+/Ca2+ exchanger (NCX) isoforms NCX1 and NCX3, we synthesized novel benzodiazepinone derivatives of the unique NCX activator Neurounina-1, named compounds 1-19. The derivatives are characterized by a benzodiazepinonic nucleus linked to five- or six-membered cyclic amines via a methylene, ethylene, or acetyl spacer. The compounds have been screened on NCX1/NCX3 isoform activities by a high-throughput screening approach, and the most promising were characterized by patch-clamp electrophysiology and Fura-2AM video imaging. We identified two novel modulators of NCX: compound 4, inhibiting NCX1 reverse mode, and compound 14, enhancing NCX1 and NCX3 activity. Compound 1 displayed neuroprotection in two preclinical models of brain ischemia. The analysis of the conformational and steric features led to the identification of the molecular volume required for selective NCX1 activation for mixed NCX1/NCX3 activation or for NCX1 inhibition, providing the first prototypal model for the design of optimized isoform modulators.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


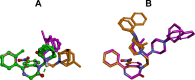

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

