Aneurysm and Artery Dissection Following the Use of Vascular Endothelial Growth Factor Inhibitor: A Real-World Analysis Using a Spontaneous Reporting System
- PMID: 34845918
- PMCID: PMC9075350
- DOI: 10.1161/JAHA.121.020844
Aneurysm and Artery Dissection Following the Use of Vascular Endothelial Growth Factor Inhibitor: A Real-World Analysis Using a Spontaneous Reporting System
Abstract
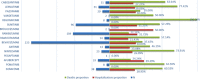
Background Pharmacological inhibition of angiogenesis via the vascular endothelial growth factor pathway is an important therapeutic target that prevents tumor growth and the formation of metastases. Although vascular endothelial growth factor inhibitor (VPI) is well understood as a well-defined safety profile, few real-world studies are comparing the incidence, clinical features, and prognosis of the aneurysm and artery dissection. Methods and Results To evaluate and compare the links between different VPIs and aneurysm and artery dissection, we identified 634 reports with VPIs in the US Food and Drug Administration Adverse Event Reporting System database ranging between January 2004 to March 2020. We used the reporting odds ratio for the association between the use of VPIs and aneurysm and artery dissection. The reporting odds ratio (3.68, 95%, 2.18‒6.23) shows that ramucirumab has a stronger correlation than other VPIs. The results show a significant difference in onset time (P<0.001). The median time to aneurysm and artery dissection was 79.5 (interquartile interval, 19.0-273.5) days after VPI administration. The results also show that VPI-associated aneurysm and artery dissection was reported more often in men (n=336, 59.68% versus n=227, 40.32%), and there were more cases in patients aged between 45 to 74 years than those <45 years (n=312, 68.12% versus n=18, 3.93%); patients aged ≥75 years accounted for 27.95% (n=128). Finally, the suspected drugs generally led to 19.98% deaths and 29.81% hospitalizations. Conclusions We identified signals for aneurysm and artery dissection following various VPIs in real-world practice via the Food and Drug Administration Adverse Event Reporting System, which represents the first step for continued pharmacovigilance investigation.
Keywords: cancer; disproportionality analysis; patient safety; pharmacovigilance; voluntary incident reporting.
Figures
Similar articles
-
Risk of artery dissection during systemic exposure to vascular endothelial growth factor pathway inhibitors.Clin Transl Sci. 2024 Dec;17(12):e70096. doi: 10.1111/cts.70096. Clin Transl Sci. 2024. PMID: 39618058 Free PMC article.
-
Arterial aneurysm and dissection with systemic vascular endothelial growth factor inhibitors: A review of cases reported to the FDA Adverse Event Reporting System and published in the literature.Vasc Med. 2021 Oct;26(5):526-534. doi: 10.1177/1358863X211006470. Epub 2021 Apr 12. Vasc Med. 2021. PMID: 33840328 Review.
-
Vascular Endothelial Growth Factor Inhibitors and the Risk of Aortic Aneurysm and Aortic Dissection.JAMA Netw Open. 2024 Mar 4;7(3):e240940. doi: 10.1001/jamanetworkopen.2024.0940. JAMA Netw Open. 2024. PMID: 38436956 Free PMC article.
-
The Association between PDE5 Inhibitors and Aneurysm/Arterial Dissection:A Pharmacovigilance Study Using WHO Safety Database.J Med Invest. 2024;71(1.2):134-140. doi: 10.2152/jmi.71.134. J Med Invest. 2024. PMID: 38735709
-
Evaluation of patient reporting of adverse drug reactions to the UK 'Yellow Card Scheme': literature review, descriptive and qualitative analyses, and questionnaire surveys.Health Technol Assess. 2011 May;15(20):1-234, iii-iv. doi: 10.3310/hta15200. Health Technol Assess. 2011. PMID: 21545758 Review.
Cited by
-
Aneurysm and Artery Dissection After Oral VEGFR-TKI Use in Adults With Cancer.JAMA Netw Open. 2023 Nov 1;6(11):e2345977. doi: 10.1001/jamanetworkopen.2023.45977. JAMA Netw Open. 2023. PMID: 38019511 Free PMC article.
-
A case of hepatocellular carcinoma with pseudoaneurysm formation upon lenvatinib administration.Clin J Gastroenterol. 2024 Apr;17(2):319-326. doi: 10.1007/s12328-023-01914-7. Epub 2024 Jan 28. Clin J Gastroenterol. 2024. PMID: 38281290
-
Aneurysm Is Restricted by CD34+ Cell-Formed Fibrous Collars Through the PDGFRb-PI3K Axis.Adv Sci (Weinh). 2025 Feb;12(7):e2408996. doi: 10.1002/advs.202408996. Epub 2024 Dec 27. Adv Sci (Weinh). 2025. PMID: 39731355 Free PMC article.
-
Acute aortic catastrophe caused by cardiovascular oncological manipulation by tyrosine kinase inhibitors with immune checkpoint blockades: a case report and literature review.Eur Heart J Case Rep. 2024 Apr 5;8(4):ytae169. doi: 10.1093/ehjcr/ytae169. eCollection 2024 Apr. Eur Heart J Case Rep. 2024. PMID: 38887778 Free PMC article.
-
Risk of artery dissection during systemic exposure to vascular endothelial growth factor pathway inhibitors.Clin Transl Sci. 2024 Dec;17(12):e70096. doi: 10.1111/cts.70096. Clin Transl Sci. 2024. PMID: 39618058 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical