Cisplatin Uptake in Macrophage Subtypes at the Single-Cell Level by LA-ICP-TOFMS Imaging
- PMID: 34846133
- PMCID: PMC8674877
- DOI: 10.1021/acs.analchem.1c03442
Cisplatin Uptake in Macrophage Subtypes at the Single-Cell Level by LA-ICP-TOFMS Imaging
Abstract
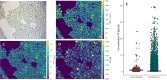
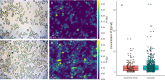
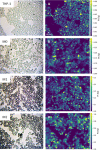
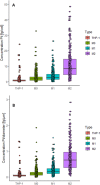
A high-throughput laser ablation-inductively coupled plasma-time-of-flight mass spectrometry (LA-ICP-TOFMS) workflow was implemented for quantitative single-cell analysis following cytospin preparation of cells. For the first time, in vitro studies on cisplatin exposure addressed human monocytes and monocyte-derived macrophages (undifferentiated THP-1 monocytic cells, differentiated M0 macrophages, as well as further polarized M1 and M2 phenotypes) at the single-cell level. The models are of particular interest as macrophages comprise the biggest part of immune cells present in the tumor microenvironment and play an important role in modulating tumor growth and progression. The introduced bioimaging workflow proved to be universally applicable to adherent and suspension cell cultures and fit-for-purpose for the quantitative analysis of several hundreds of cells within minutes. Both, cross-validation of the method with single-cell analysis in suspension for THP-1 cells and with LA-ICP-TOFMS analysis of adherent M0 cells grown on chambered glass coverslips, revealed agreeing platinum concentrations at the single-cell level. A high incorporation of cisplatin was observed in M2 macrophages compared to the M0 and M1 macrophage subtypes and the monocyte model, THP-1. The combination with bright-field images and monitoring of highly abundant endogenous elements such as phosphorus and sodium at a high spatial resolution allowed assessing cell size and important morphological cell parameters and thus straightforward control over several cell conditions. This way, apoptotic cells and cell debris as well as doublets or cell clusters could be easily excluded prior to data evaluation without additional staining.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Theiner S.; Loehr K.; Koellensperger G.; Mueller L.; Jakubowski N. Single-Cell Analysis by Use of ICP-MS. J. Anal. At. Spectrom. 2020, 35, 1784–1813. 10.1039/D0JA00194E. - DOI
-
- Zheng L.-N.; Wang M.; Zhao L.-C.; Sun B.-Y.; Wang B.; Chen H.-Q.; Zhao Y.-L.; Chai Z.-F.; Feng W.-Y. Quantitative Analysis of Gd@C82(OH)22 and Cisplatin Uptake in Single Cells by Inductively Coupled Plasma Mass Spectrometry. Anal. Bioanal. Chem. 2015, 407, 2383–2391. 10.1007/s00216-014-8422-3. - DOI - PubMed
-
- López-Serrano Oliver A.; Baumgart S.; Bremser W.; Flemig S.; Wittke D.; Grützkau A.; Luch A.; Haase A.; Jakubowski N. Quantification of Silver Nanoparticles Taken up by Single Cells Using Inductively Coupled Plasma Mass Spectrometry in the Single Cell Measurement Mode. J. Anal. At. Spectrom. 2018, 33, 1256–1263. 10.1039/C7JA00395A. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

