Negative X-ray expansion in cadmium cyanide
- PMID: 34846452
- PMCID: PMC8111741
- DOI: 10.1039/d0mh01989e
Negative X-ray expansion in cadmium cyanide
Abstract
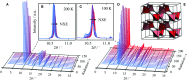
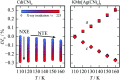
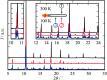
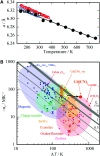
Cadmium cyanide, Cd(CN)2, is a flexible coordination polymer best studied for its strong and isotropic negative thermal expansion (NTE) effect. Here we show that this NTE is actually X-ray-exposure dependent: Cd(CN)2 contracts not only on heating but also on irradiation by X-rays. This behaviour contrasts that observed in other beam-sensitive materials, for which X-ray exposure drives lattice expansion. We call this effect 'negative X-ray expansion' (NXE) and suggest its origin involves an interaction between X-rays and cyanide 'flips'; in particular, we rule out local heating as a possible mechanism. Irradiation also affects the nature of a low-temperature phase transition. Our analysis resolves discrepancies in NTE coefficients reported previously on the basis of X-ray diffraction measurements, and we establish the 'true' NTE behaviour of Cd(CN)2 across the temperature range 150-750 K. The interplay between irradiation and mechanical response in Cd(CN)2 highlights the potential for exploiting X-ray exposure in the design of functional materials.
Conflict of interest statement
There are no conflicts to declare.
Figures
References
-
- Blake C. C. F. and Phillips D. C., Proceedings of the Symposium on the Biological Effects of Ionizing Radiation at the Molecular Level, Int. Atomic Energy Agency, Vienna, 1962, pp. 183–191
-
- Helliwell J. R. J. Cryst. Growth. 1988;90:259–272. doi: 10.1016/0022-0248(88)90322-3. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

