Wt1 haploinsufficiency induces browning of epididymal fat and alleviates metabolic dysfunction in mice on high-fat diet
- PMID: 34846543
- PMCID: PMC8803700
- DOI: 10.1007/s00125-021-05621-1
Wt1 haploinsufficiency induces browning of epididymal fat and alleviates metabolic dysfunction in mice on high-fat diet
Abstract
Aims/hypothesis: Despite a similar fat storing function, visceral (intra-abdominal) white adipose tissue (WAT) is detrimental, whereas subcutaneous WAT is considered to protect against metabolic disease. Recent findings indicate that thermogenic genes, expressed in brown adipose tissue (BAT), can be induced primarily in subcutaneous WAT. Here, we investigate the hypothesis that the Wilms tumour gene product (WT1), which is expressed in intra-abdominal WAT but not in subcutaneous WAT and BAT, suppresses a thermogenic program in white fat cells.
Methods: Heterozygous Wt1 knockout mice and their wild-type littermates were examined in terms of thermogenic and adipocyte-selective gene expression. Glucose tolerance and hepatic lipid accumulation in these mice were assessed under normal chow and high-fat diet conditions. Pre-adipocytes isolated from the stromal vascular fraction of BAT were transduced with Wt1-expressing retrovirus, induced to differentiate and analysed for the expression of thermogenic and adipocyte-selective genes.
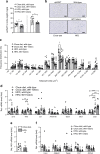
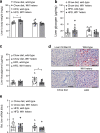
Results: Expression of the thermogenic genes Cpt1b and Tmem26 was enhanced and transcript levels of Ucp1 were on average more than tenfold higher in epididymal WAT of heterozygous Wt1 knockout mice compared with wild-type mice. Wt1 heterozygosity reduced epididymal WAT mass, improved whole-body glucose tolerance and alleviated severe hepatic steatosis upon diet-induced obesity in mice. Retroviral expression of WT1 in brown pre-adipocytes, which lack endogenous WT1, reduced mRNA levels of Ucp1, Ppargc1a, Cidea, Prdm16 and Cpt1b upon in vitro differentiation by 60-90%. WT1 knockdown in epididymal pre-adipocytes significantly lowered Aldh1a1 and Zfp423 transcripts, two key suppressors of the thermogenic program. Conversely, Aldh1a1 and Zfp423 mRNA levels were increased approximately five- and threefold, respectively, by retroviral expression of WT1 in brown pre-adipocytes.
Conclusion/interpretation: WT1 functions as a white adipocyte determination factor in epididymal WAT by suppressing thermogenic genes. Reducing Wt1 expression in this and other intra-abdominal fat depots may represent a novel treatment strategy in metabolic disease.
Keywords: Adipocyte; Browning; Glucose tolerance; Hepatic steatosis; High-fat diet; Obesity; Thermogenesis; Transcription factor; UCP1; WT1.
© 2021. The Author(s).
Figures




Similar articles
-
WT1 in Adipose Tissue: From Development to Adult Physiology.Front Cell Dev Biol. 2022 Mar 16;10:854120. doi: 10.3389/fcell.2022.854120. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35372335 Free PMC article. Review.
-
GQ-16, a TZD-Derived Partial PPARγ Agonist, Induces the Expression of Thermogenesis-Related Genes in Brown Fat and Visceral White Fat and Decreases Visceral Adiposity in Obese and Hyperglycemic Mice.PLoS One. 2016 May 3;11(5):e0154310. doi: 10.1371/journal.pone.0154310. eCollection 2016. PLoS One. 2016. PMID: 27138164 Free PMC article.
-
Overexpression of Adiponectin Receptor 1 Inhibits Brown and Beige Adipose Tissue Activity in Mice.Int J Mol Sci. 2021 Jan 18;22(2):906. doi: 10.3390/ijms22020906. Int J Mol Sci. 2021. PMID: 33477525 Free PMC article.
-
Adipocyte-specific Hypoxia-inducible gene 2 promotes fat deposition and diet-induced insulin resistance.Mol Metab. 2016 Sep 28;5(12):1149-1161. doi: 10.1016/j.molmet.2016.09.009. eCollection 2016 Dec. Mol Metab. 2016. PMID: 27900258 Free PMC article.
-
Adipocyte lineages: tracing back the origins of fat.Biochim Biophys Acta. 2014 Mar;1842(3):340-51. doi: 10.1016/j.bbadis.2013.05.027. Epub 2013 Jun 4. Biochim Biophys Acta. 2014. PMID: 23747579 Free PMC article. Review.
Cited by
-
Regulation of Mesothelial Cell Fate during Development and Human Diseases.Int J Mol Sci. 2022 Oct 8;23(19):11960. doi: 10.3390/ijms231911960. Int J Mol Sci. 2022. PMID: 36233262 Free PMC article. Review.
-
GDF15 is required for cold-induced thermogenesis and contributes to improved systemic metabolic health following loss of OPA1 in brown adipocytes.Elife. 2023 Oct 11;12:e86452. doi: 10.7554/eLife.86452. Elife. 2023. PMID: 37819027 Free PMC article.
-
Comprehensive prognostic gene identification and functional characterization of GRAMD1A in Wilms tumor: development of risk prediction models and therapeutic implications.Front Oncol. 2024 Nov 26;14:1501718. doi: 10.3389/fonc.2024.1501718. eCollection 2024. Front Oncol. 2024. PMID: 39659787 Free PMC article.
-
WT1 in Adipose Tissue: From Development to Adult Physiology.Front Cell Dev Biol. 2022 Mar 16;10:854120. doi: 10.3389/fcell.2022.854120. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35372335 Free PMC article. Review.
-
Systematic functional characterization of non-coding regulatory SNPs associated with central obesity.Am J Hum Genet. 2025 Jan 2;112(1):116-134. doi: 10.1016/j.ajhg.2024.11.005. Am J Hum Genet. 2025. PMID: 39753113 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

