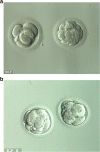
Live birth after in vitro maturation in women with gonadotropin resistance ovary syndrome: report of two cases
- PMID: 34846627
- PMCID: PMC8666402
- DOI: 10.1007/s10815-021-02355-2
Live birth after in vitro maturation in women with gonadotropin resistance ovary syndrome: report of two cases
Abstract
Purpose: Gonadotropin-resistant ovary syndrome (GROS) is a rare endocrine disorder that causes hypergonadotropic hypogonadism, amenorrhea, and infertility. This study reports live birth in two women with GROS who underwent fertility treatment with in vitro maturation (IVM).
Methods: Both patients had primary infertility, amenorrhea (primary and secondary), typical secondary sexual characters, elevated gonadotropin levels, normal ovarian reserve, normal chromosomal characteristics, and previous nonresponsiveness gonadotropin stimulations. One patient had polymorphism of the follicle-stimulating hormone receptor, which is a predictor of poor ovarian response. Given unresponsiveness to exogenous gonadotropin stimulations, IVM with human chorionic gonadotropin priming (hCG-IVM) was performed in both patients. All transferrable embryos were vitrified.
Results: Both patients achieved pregnancy after their first frozen embryos transfer, and each delivered a healthy baby boy.
Conclusions: These results suggest that IVM should be a first-line therapeutic option for patients with GROS.
Keywords: Gonadotropin-resistant ovary syndrome; In vitro maturation; Infertility; Live birth.
© 2021. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
LNV has received speaker and conference fees from Merck; and grant, speaker, and conference fees from Merck Sharpe & Dohme and Ferring. TMH has received speaker fees from Merck, Merck Sharp & Dohme, and Ferring. All other authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical