State of the art treatment of hepatitis B virus hepatocellular carcinoma and the role of hepatitis B surface antigen post-liver transplantation and resection
- PMID: 34846790
- PMCID: PMC9300017
- DOI: 10.1111/liv.15124
State of the art treatment of hepatitis B virus hepatocellular carcinoma and the role of hepatitis B surface antigen post-liver transplantation and resection
Abstract
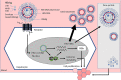
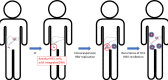
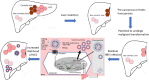
Chronic hepatitis B virus (HBV) infection is the major aetiology of hepatocellular carcinoma (HCC). The optimal goal of therapy, hepatitis B surface antigen (HBsAg) loss and anti-HBs production, is achieved rarely and HBsAg-associated HCC risk is well recognized. Here we review the role of HBsAg in HCC, the link between HBsAg and HCC recurrence post-liver transplantation or resection, and the implications for therapy. HBV-associated carcinogenesis is a multifactorial process. The observation that HBV-related HCC can occur in the absence of cirrhosis is compatible with a direct oncogenic effect of the virus, which may occur via multiple mechanisms, including those mediated by both mutated and unmutated HBsAg. HCC recurrence in HBsAg-positive patients post-liver transplantation has been reported in 10%-15% of patients and is likely to be because of expansion of residual HCC tumour cell populations containing integrated HBV DNA, which expand and independently replicate HBV, leading to the recurrence of both HCC and HBV. The direct role of HBsAg in HCC recurrence post-liver resection is less clear. Cirrhosis is the most important risk factor for HCC development, and precancerous cirrhotic liver remains after resection, with the potential to undergo malignant transformation regardless of the existence of HBV-derived oncogenic drivers. The role of HBsAg in the development of HCC and its recurrence post-surgical intervention has multiple implications for therapy and suggests a potential role for immunotherapy in the future management of HCC, in particular post-liver transplantation. Use of hepatitis B immunoglobulins that target HBsAg directly, alongside immune-oncology therapies, may be relevant in this setting.
Keywords: HBsAg; hepatitis B; hepatocellular carcinoma; resection; transplantation.
© 2021 The Authors. Liver International published by John Wiley & Sons Ltd.
Conflict of interest statement
Authors disclose the following potential conflict of interest in the last 5 years. Related to manuscript topic: PS, PB, R‐HH, CMH, CL, KM, AV, DS; Biotest AG. Unrelated to manuscript topic: PS: Allergosan, ArgosMed, Astellas, Bard Medical, Baxter, Chiesi, Dr Fanz Köhler Chemie, Ethicon, Hexal, Medtronic, Merck, Neovii, Novartis, Panacea Biotec, Sandoz, Sanofi‐Aventis GmbH, Shire, TEVA‐ratiopharm; PB: Biotest, Kedrion, Chiesi Farmaceutici; R‐HH: No conflict of interest declared; CMH: Employee of Biotest AG; CL: Astellas, Novartis, Grand Fontaine, MSD, Ethicon, Medtronic, Roche; KM: Biotest AG; AV: AstraZeneca, Bayer, Bristol‐Myers Squibb, Eisai, Eli Lilly, Incyte Corporation, Ipsen, Merck, MSD, Pierre Fabre, Roche, Sanofi; DS: Astellas, Go liver, Gilead Sciences.
Figures

References
-
- EASL Clinical Practice Guidelines . Management of hepatocellular carcinoma. J Hepatol. 2018;69:182‐236. - PubMed
-
- Akinyemiju T, Abera S, Ahmed M, et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level results from the global burden of disease study 2015 global burden of disease liver cancer collaboration. JAMA Oncol. 2017;3:1683‐1691. - PMC - PubMed
-
- EASL Clinical Practice Guidelines . Management of hepatitis B virus infection. J Hepatol. 2017;67:370‐398. - PubMed
-
- WHO Global hepatitis report; 2017. https://www.who.int/hepatitis/publications/global‐hepatitis‐report2017/en/
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

