Oxidized Phospholipids Promote NETosis and Arterial Thrombosis in LNK(SH2B3) Deficiency
- PMID: 34846914
- PMCID: PMC8663540
- DOI: 10.1161/CIRCULATIONAHA.121.056414
Oxidized Phospholipids Promote NETosis and Arterial Thrombosis in LNK(SH2B3) Deficiency
Erratum in
-
Correction to: Oxidized Phospholipids Promote NETosis and Arterial Thrombosis in LNK(SH2B3) Deficiency.Circulation. 2022 Jul 26;146(4):e13. doi: 10.1161/CIR.0000000000001087. Epub 2022 Jul 25. Circulation. 2022. PMID: 35877838 Free PMC article. No abstract available.
Abstract
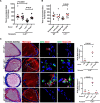
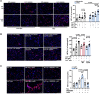
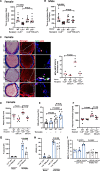
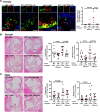
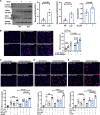
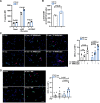
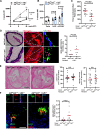
Background: LNK/SH2B3 inhibits Janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling by hematopoietic cytokine receptors. Genome-wide association studies have shown association of a common single nucleotide polymorphism in LNK (R262W, T allele) with neutrophilia, thrombocytosis, and coronary artery disease. We have shown that LNK(TT) reduces LNK function and that LNK-deficient mice display prominent platelet-neutrophil aggregates, accelerated atherosclerosis, and thrombosis. Platelet-neutrophil interactions can promote neutrophil extracellular trap (NET) formation. The goals of this study were to assess the role of NETs in atherosclerosis and thrombosis in mice with hematopoietic Lnk deficiency.
Methods: We bred mice with combined deficiency of Lnk and the NETosis-essential enzyme PAD4 (peptidyl arginine deiminase 4) and transplanted their bone marrow into Ldlr-/- mice. We evaluated the role of LNK in atherothrombosis in humans and mice bearing a gain of function variant in JAK2 (JAK2V617F).
Results: Lnk-deficient mice displayed accelerated carotid artery thrombosis with prominent NETosis that was completely reversed by PAD4 deficiency. Thrombin-activated Lnk-/- platelets promoted increased NETosis when incubated with Lnk-/- neutrophils compared with wild-type platelets or wild-type neutrophils. This involved increased surface exposure and release of oxidized phospholipids (OxPL) from Lnk-/- platelets, as well as increased priming and response of Lnk-/- neutrophils to OxPL. To counteract the effects of OxPL, we introduced a transgene expressing the single-chain variable fragment of E06 (E06-scFv). E06-scFv reversed accelerated NETosis, atherosclerosis, and thrombosis in Lnk-/- mice. We also showed increased NETosis when human induced pluripotent stem cell-derived LNK(TT) neutrophils were incubated with LNK(TT) platelet/megakaryocytes, but not in isogenic LNK(CC) controls, confirming human relevance. Using data from the UK Biobank, we found that individuals with the JAK2VF mutation only showed increased risk of coronary artery disease when also carrying the LNK R262W allele. Mice with hematopoietic Lnk+/- and Jak2VF clonal hematopoiesis showed accelerated arterial thrombosis but not atherosclerosis compared with Jak2VFLnk+/+ controls.
Conclusions: Hematopoietic Lnk deficiency promotes NETosis and arterial thrombosis in an OxPL-dependent fashion. LNK(R262W) reduces LNK function in human platelets and neutrophils, promoting NETosis, and increases coronary artery disease risk in humans carrying Jak2VF mutations. Therapies targeting OxPL may be beneficial for coronary artery disease in genetically defined human populations.
Keywords: clonal hematopoiesis; coronary artery disease; extracellular traps; phospholipids; thrombosis.
Figures
References
-
- Deloukas P, Kanoni S, Willenborg C, Farrall M, Assimes TL, Thompson JR, Ingelsson E, Saleheen D, Erdmann J, Goldstein BA, et al. ; CARDIoGRAMplusC4D Consortium; DIAGRAM Consortium; CARDIOGENICS Consortium; MuTHER Consortium; Wellcome Trust Case Control Consortium. Large-scale association analysis identifies new risk loci for coronary artery disease. Nat Genet. 2013; 45:25–33. doi: 10.1038/ng.2480 - PMC - PubMed
-
- Gudbjartsson DF, Bjornsdottir US, Halapi E, Helgadottir A, Sulem P, Jonsdottir GM, Thorleifsson G, Helgadottir H, Steinthorsdottir V, Stefansson H, et al. Sequence variants affecting eosinophil numbers associate with asthma and myocardial infarction. Nat Genet. 2009; 41:342–347. doi: 10.1038/ng.323 - PubMed
-
- Barrett JC, Clayton DG, Concannon P, Akolkar B, Cooper JD, Erlich HA, Julier C, Morahan G, Nerup J, Nierras C, et al. ; Type 1 Diabetes Genetics Consortium. Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat Genet. 2009; 41:703–707. doi: 10.1038/ng.381 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

