Nasal continuous positive airway pressure levels for the prevention of morbidity and mortality in preterm infants
- PMID: 34847243
- PMCID: PMC8631577
- DOI: 10.1002/14651858.CD012778.pub2
Nasal continuous positive airway pressure levels for the prevention of morbidity and mortality in preterm infants
Abstract
Background: Preterm infants are at risk of lung atelectasis due to various anatomical and physiological immaturities, placing them at high risk of respiratory failure and associated harms. Nasal continuous positive airway pressure (CPAP) is a positive pressure applied to the airways via the nares. It helps prevent atelectasis and supports adequate gas exchange in spontaneously breathing infants. Nasal CPAP is used in the care of preterm infants around the world. Despite its common use, the appropriate pressure levels to apply during nasal CPAP use remain uncertain.
Objectives: To assess the effects of 'low' (≤ 5 cm H2O) versus 'moderate-high' (> 5 cm H2O) initial nasal CPAP pressure levels in preterm infants receiving CPAP either: 1) for initial respiratory support after birth and neonatal resuscitation or 2) following mechanical ventilation and endotracheal extubation.
Search methods: We ran a comprehensive search on 6 November 2020 in the following databases: CENTRAL via CRS Web and MEDLINE via Ovid. We also searched clinical trials databases and the reference lists of retrieved articles for randomized controlled trials (RCTs) and quasi-randomized trials.
Selection criteria: We included RCTs, quasi-RCTs, cluster-RCTs and cross-over RCTs randomizing preterm infants of gestational age < 37 weeks or birth weight < 2500 grams within the first 28 days of life to different nasal CPAP levels.
Data collection and analysis: We used the standard methods of Cochrane Neonatal to collect and analyze data. We used the GRADE approach to assess the certainty of the evidence for the prespecified primary outcomes.
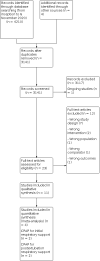
Main results: Eleven trials met inclusion criteria of the review. Four trials were parallel-group RCTs reporting our prespecified primary or secondary outcomes. Two trials randomized 316 infants to low versus moderate-high nasal CPAP for initial respiratory support, and two trials randomized 117 infants to low versus moderate-high nasal CPAP following endotracheal extubation. The remaining seven studies were cross-over trials reporting short-term physiological outcomes. The most common potential sources of bias were absent or unclear blinding of personnel and assessors and uncertain selective reporting. Nasal CPAP for initial respiratory support after birth and neonatal resuscitation None of the six primary outcomes prespecified for inclusion in the summary of findings was eligible for meta-analysis. No trials reported on moderate-severe neurodevelopmental impairment at 18 to 26 months. The remaining five outcomes were reported in a single trial. On the basis of this trial, we are uncertain whether low or moderate-high nasal CPAP levels improve the outcomes of: death or bronchopulmonary dysplasia (BPD) at 36 weeks' postmenstrual age (PMA) (risk ratio (RR) 1.02, 95% confidence interval (CI) 0.56 to 1.85; 1 trial, 271 participants); mortality by hospital discharge (RR 1.04, 95% CI 0.51 to 2.12; 1 trial, 271 participants); BPD at 28 days of age (RR 1.10, 95% CI 0.56 to 2.17; 1 trial, 271 participants); BPD at 36 weeks' PMA (RR 0.80, 95% CI 0.25 to 2.57; 1 trial, 271 participants), and treatment failure or need for mechanical ventilation (RR 1.00, 95% CI 0.63 to 1.57; 1 trial, 271 participants). We assessed the certainty of the evidence as very low for all five outcomes due to risk of bias, a lack of consistency across multiple studies, and imprecise effect estimates. Nasal CPAP following mechanical ventilation and endotracheal extubation One of the six primary outcomes prespecified for inclusion in the summary of findings was eligible for meta-analysis. On the basis of these data, we are uncertain whether low or moderate-high nasal CPAP levels improve the outcome of treatment failure or need for mechanical ventilation (RR 1.52, 95% CI 0.92 to 2.50; 2 trials, 117 participants; I2 = 17%; risk difference 0.15, 95% CI -0.02 to 0.32; number needed to treat for an additional beneficial outcome 7, 95% CI -50 to 3). We assessed the certainty of the evidence as very low due to risk of bias, inconsistency across the studies, and imprecise effect estimates. No trials reported on moderate-severe neurodevelopmental impairment at 18 to 26 months or BPD at 28 days of age. The remaining three outcomes were reported in a single trial. On the basis of this trial, we are uncertain whether low or moderate-high nasal CPAP levels improve the outcomes of: death or BPD at 36 weeks' PMA (RR 0.87, 95% CI 0.51 to 1.49; 1 trial, 93 participants); mortality by hospital discharge (RR 2.94, 95% CI 0.12 to 70.30; 1 trial, 93 participants), and BPD at 36 weeks' PMA (RR 0.87, 95% CI 0.51 to 1.49; 1 trial, 93 participants). We assessed the certainty of the evidence as very low for all three outcomes due to risk of bias, a lack of consistency across multiple studies, and imprecise effect estimates. AUTHORS' CONCLUSIONS: There are insufficient data from randomized trials to guide nasal CPAP level selection in preterm infants, whether provided as initial respiratory support or following extubation from invasive mechanical ventilation. We are uncertain as to whether low or moderate-high nasal CPAP levels improve morbidity and mortality in preterm infants. Well-designed trials evaluating this important aspect of a commonly used neonatal therapy are needed.
Copyright © 2021 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
N Bamat is employed as a neonatologist, Children's Hospital of Philadelphia.
J Fierro has no interest to declare.
A Mukerji has no interest to declare.
CJ Wright is an Associate Professor, Section of Neonatology, University of Colorado.
D Millar has received honoraria teaching neonatal trainees from Chiesi Ltd. Dr Millar is employed as a Consultant Neonatologist, Belfast Health & Social Care Trust.
H Kirpalani has no interest to declare.
Figures




























Update of
References
References to studies included in this review
Beker 2014 {published data only}
-
- Beker F, Rogerson SR, Hooper SB, Wong C, Davis PG. The effects of nasal continuous positive airway pressure on cardiac function in premature infants with minimal lung disease: a crossover randomized trial. Journal of Pediatrics 2014;164(4):726-9. [DOI: 10.1016/j.jpeds.2013.10.087] [PMID: ] - DOI - PubMed
Buzzella 2014 {published data only (unpublished sought but not used)}
Dhir 2016 {published and unpublished data}
Elgellab 2001 {published data only}
-
- Elgellab A, Riou Y, Abbazine A, Truffert P, Matran R, Lequien P, et al. Effects of nasal continuous positive airway pressure (NCPAP) on breathing pattern in spontaneously breathing premature newborn infants. Intensive Care Medicine 2001;27(11):1782-7. [DOI: 10.1007/s00134-001-1117-1] [PMID: ] - DOI - PubMed
Kitsommart 2013 {published and unpublished data}
-
- Kitsommart R, Kalyn A, Al-Saleem N, Janes M, Sant'Anna GM. Levels of nasal CPAP applied during the immediate post-extubation phase: a randomized controlled pilot trial. e-Journal of Neonatology Research 2013;3(1):2-10.
Lavizzari 2014 {published and unpublished data}
Lomp 2010 {unpublished data only}
-
- Lomp A. Measurement and assessment of work of breathing in neonates during nasal continuous positive airway pressure therapy. Thesis submitted for MDres degree, Department of Paediatrics, Imperial College London (spiral.imperial.ac.uk/bitstream/10044/1/7019/1/Lomp-A-2011-MD%28Res%29-T...) 2010.
Magnenant 2004 {published data only}
-
- Magnenant E, Rakza T, Riou Y, Elgellab A, Matran R, Lequien P, et al. Dynamic behavior of respiratory system during nasal continuous positive airway pressure in spontaneously breathing premature newborn infants. Pediatric Pulmonology 2004;37(6):485-91. [DOI: 10.1002/ppul.10445] [PMID: ] - DOI - PubMed
Miedema 2013 {published data only (unpublished sought but not used)}
Murki 2016 {published and unpublished data}
-
- Murki S, Nathani PP, Sharma D, Subramaniam S, Oleti TP, Chawla D. Initiating nasal continuous positive airway pressure in preterm neonates at 5 cm as against 7 cm did not decrease the need for mechanical ventilation. Acta Paediatrica 2016;105(8):e345-51. [DOI: 10.1111/apa.13385] [PMID: ] - DOI - PubMed
References to studies excluded from this review
Beker 2015 {published data only}
-
- Beker F, Rogerson SR, Hooper SB, Sehgal A, Davis PG. Hemodynamic effects of nasal continuous positive airway pressure in preterm infants with evolving chronic lung disease, a crossover randomized trial. Journal of Pediatrics 2015;166(2):477-9. [DOI: 10.1016/j.jpeds.2014.10.019] [PMID: ] - DOI - PubMed
Courtney 2003 {published data only}
-
- Courtney SE, Aghai ZH, Saslow JG, Pyon KH, Habib RH. Changes in lung volume and work of breathing: a comparison of two variable-flow nasal continuous positive airway pressure devices in very low birth weight infants. Pediatric Pulmonology 2003;36(3):248-52. [DOI: 10.1002/ppul.10327] [PMID: ] - DOI - PubMed
Courtney 2011 {published data only}
Dimitriou 1999 {published data only}
Dinger 2001 {published data only}
Lavizzari 2016 {published data only}
-
- Lavizzari A, Colnaghi M, Ciuffini F, Veneroni C, Musumeci S, Cortinovis I, et al. Heated, humidified high-flow nasal cannula vs nasal continuous positive airway pressure for respiratory distress syndrome of prematurity: a randomized clinical noninferiority trial. JAMA Pediatrics August 8, 2016:E1-E7. [DOI: 10.1001/jamapediatrics.2016.1243] [PMID: ] - DOI - PubMed
Locke 1991 {published data only}
Mukerji 2019 {published data only}
Pandit 2001 {published data only}
Pickerd 2014 {published data only}
Veneroni 2014 {published data only}
-
- Veneroni C, Zannin E, Ventura L, Colombo M, Tagliabue P, Suki B, et al. Effect of nasal continuous positive airways pressure (NCPAP) on variability in breathing pattern of preterm newborns. American Journal of Respiratory and Critical Care Medicine 2014;189:A2605.
References to ongoing studies
ACTRN12618001638224 {published data only}
-
- ACTRN12618001638224. Extubation CPAP Level Assessment trial (ÉCLAT) [A multicentre randomised control trial comparing high (9-11 cmH2O) vs. standard (6-8 cmH2O) Continuous Positive Airway Pressures after Extubation in Extremely Preterm Infants to reduce extubation failure]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=375912 (first received 25 September 2018).
Additional references
Abdel‐Hady 2008
Bayley 1993
-
- Bayley N. Bayley Scales of Infant Development. 2nd edition. San Antonio (TX): The Psychological Corporation, 1993.
Bayley 2006
-
- Bayley N. Bayley Scales of Infant and Toddler Development. San Antonio (TX): Harcourt Assessment, 2006.
Bell 1978
Covidence [Computer program]
-
- Veritas Health Innovation Covidence systematic review software. Melbourne, Australia: Veritas Health Innovation, 2021. Available at www.covidence.org.
Danan 2008
Davis 2003
De Paoli 2008
de Vries 1992
Ehrenkranz 2005
-
- Ehrenkranz RA, Walsh MC, Vohr BR, Jobe AH, Wright LL, Fanaroff AA, et al, National Institute of Child Health and Human Development Neonatal Research Network. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics 2005;116(6):1353-60. [DOI: 10.1542/peds.2005-0249] [PMID: ] - DOI - PubMed
Fajardo 2014
Fischer 2013
Göpel 2011
-
- Göpel W, Kribs A, Ziegler A, Laux R, Hoehn T, Wieg C, et al, German Neonatal Network. Avoidance of mechanical ventilation by surfactant treatment of spontaneously breathing preterm infants (AMV): an open-label, randomised, controlled trial. Lancet 2011;378(9803):1627-34. [DOI: 10.1016/S0140-6736(11)60986-0] [PMID: ] - DOI - PubMed
GRADEpro GDT [Computer program]
-
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed 1 September 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2014. Available at gradepro.org.
Gregory 1971
Griffiths 1954
-
- Griffiths R. The Abilities of Babies: A Study in Mental Measurement. New York (NY): McGraw-Hill Book Co., Inc., 1954.
Higgins 2011
-
- Higgins JP, Altman DG, Sterne JA: on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2021
-
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook. - PMC - PubMed
Ho 2015
ICROP 2005
Jacobs 2013
Jobe 2001
Lemyre 2016
-
- Lemyre B, Laughon M, Bose C, Davis PG. Early nasal intermittent positive pressure ventilation (NIPPV) versus early nasal continuous positive airway pressure (NCPAP) for preterm infants. Cochrane Database of Systematic Reviews 2016, Issue 12. Art. No: CD005384. [DOI: 10.1002/14651858.CD005384.pub2] - DOI - PMC - PubMed
Lemyre 2017
-
- Lemyre B, Davis PG, De Paoli AG, Kirpalani H. Nasal intermittent positive pressure ventilation (NIPPV) versus nasal continuous positive airway pressure (NCPAP) for preterm neonates after extubation. Cochrane Database of Systematic Reviews 2017, Issue 2. Art. No: CD003212. [DOI: 10.1002/14651858.CD003212.pub3] - DOI - PMC - PubMed
Martin 1977
Miller 1990
Milner 1977
Morley 2008
Muller 1979
NCT04372953
-
- NCT04372953. Positive End-Expiratory Pressure (PEEP) Levels During Resuscitation of Preterm Infants at Birth (The POLAR Trial). clinicaltrials.gov/ct2/show/NCT04372953 (first received 4 May 2020).
NIH 1979
-
- National Institutes of Health. Report of Workshop on Bronchopulmonary Dysplasia. In: NIH Publication No. 80-1660. Washington, DC, 1979.
Palisano 1997
Papile 1978
Patel 2015
-
- Patel RM, Kandefer S, Walsh MC, Bell EF, Carlo WA, Laptook AR, et al, Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Causes and timing of death in extremely premature infants from 2000 through 2011. New England Journal of Medicine 2015;372(4):331-40. [DOI: 10.1056/NEJMoa1403489] [PMID: ] - DOI - PMC - PubMed
RevMan Web 2019 [Computer program]
-
- The Cochrane Collaboration Review Manager Web (RevMan Web). The Cochrane Collaboration, 2019. Available at revman.cochrane.org.
Schmölzer 2013
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Shennan 1988
-
- Shennan AT, Dunn MS, Ohlsson A, Lennox K, Hoskins EM. Abnormal pulmonary outcomes in premature infants: prediction from oxygen requirement in the neonatal period. Pediatrics 1988;82(4):527-32. [PMID: ] - PubMed
SUPPORT 2010
-
- SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network, Finer NN, Carlo WA, Walsh MC, Rich W, Gantz MG, et al. Early CPAP versus surfactant in extremely preterm infants. New England Journal of Medicine 2010;362(21):1970-9. Erratum in N Engl J Med. 2010 Jun 10;362(23):2235. [DOI: 10.1056/NEJMoa0911783] [PMID: ] - DOI - PMC - PubMed
Ueda 1994
Waitz 2020
-
- Waitz M, Engel C, Schloesser R, Rochwalsky U, Meyer S, Zemlin M, et al. Application of two different nasal CPAP levels for the treatment of respiratory distress syndrome in preterm infants —“The OPTTIMMAL-Trial”—Optimizing PEEP To The IMMAture Lungs: study protocol of a randomized controlled trial. Trials 2020;21:822. [DOI: 10.1186/s13063-020-04660-0] [PMID: ] - DOI - PMC - PubMed
Walsh 2004
-
- Walsh MC, Yao Q, Gettner P, Hale E, Collins M, Hensman A, et al, National Institute of Child Health and Human Development Neonatal Research Network. Impact of a physiologic definition on bronchopulmonary dysplasia rates. Pediatrics 2004;114:1305-11. [DOI: 10.1542/peds.2004-0204] [PMID: ] - DOI - PubMed
Wheeler 2011
World Bank 2021
-
- World Bank. World Bank Country and Lending Groups. datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-coun... (accessed 4 May 2021).
References to other published versions of this review
Bamat 2017
-
- Bamat N, Fierro J, Wright CJ, Millar D, Kirpalani H. Nasal continuous positive airway pressure levels for the prevention of morbidity and mortality in very low birth weight infants. Cochrane Database of Systematic Reviews 2017, Issue 9. Art. No: CD012778. [DOI: 10.1002/14651858.CD012778] - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

