Current Advances of Artificial Pancreas Systems: A Comprehensive Review of the Clinical Evidence
- PMID: 34847641
- PMCID: PMC8640161
- DOI: 10.4093/dmj.2021.0177
Current Advances of Artificial Pancreas Systems: A Comprehensive Review of the Clinical Evidence
Abstract
Since Banting and Best isolated insulin in the 1920s, dramatic progress has been made in the treatment of type 1 diabetes mellitus (T1DM). However, dose titration and timely injection to maintain optimal glycemic control are often challenging for T1DM patients and their families because they require frequent blood glucose checks. In recent years, technological advances in insulin pumps and continuous glucose monitoring systems have created paradigm shifts in T1DM care that are being extended to develop artificial pancreas systems (APSs). Numerous studies that demonstrate the superiority of glycemic control offered by APSs over those offered by conventional treatment are still being published, and rapid commercialization and use in actual practice have already begun. Given this rapid development, keeping up with the latest knowledge in an organized way is confusing for both patients and medical staff. Herein, we explore the history, clinical evidence, and current state of APSs, focusing on various development groups and the commercialization status. We also discuss APS development in groups outside the usual T1DM patients and the administration of adjunct agents, such as amylin analogues, in APSs.
Keywords: Blood glucose self-monitoring; Diabetes mellitus, type 1; Hypoglycemia; Insulin infusion systems; Pancreas, artificial; Wearable electronic devices.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures
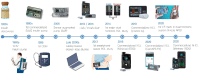
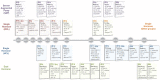
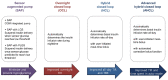


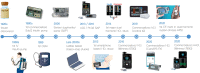
References
-
- SEARCH for Diabetes in Youth Study Group. Liese AD, D’Agostino RB, Jr, Hamman RF, Kilgo PD, Lawrence JM, et al. The burden of diabetes mellitus among US youth: prevalence estimates from the SEARCH for Diabetes in Youth Study. Pediatrics. 2006;118:1510–8. - PubMed
-
- Dayan CM, Korah M, Tatovic D, Bundy BN, Herold KC. Changing the landscape for type 1 diabetes: the first step to prevention. Lancet. 2019;394:1286–96. - PubMed
-
- Dean PG, Kukla A, Stegall MD, Kudva YC. Pancreas transplantation. BMJ. 2017;357:j1321. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

