Synaptic pathology in Huntington's disease: Beyond the corticostriatal pathway
- PMID: 34848336
- PMCID: PMC9328779
- DOI: 10.1016/j.nbd.2021.105574
Synaptic pathology in Huntington's disease: Beyond the corticostriatal pathway
Abstract
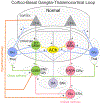
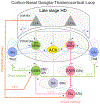
Huntington's disease (HD) is a heritable, fatal neurodegenerative disorder caused by a mutation in the Huntingtin gene. It is characterized by chorea, as well as cognitive and psychiatric symptoms. Histopathologically, there is a massive loss of striatal projection neurons and less but significant loss in other areas throughout the cortico-basal ganglia-thalamocortical (CBGTC) loop. The mutant huntingtin protein has been implicated in numerous functions, including an important role in synaptic transmission. Most studies on anatomical and physiological alterations in HD have focused on striatum and cerebral cortex. However, based on recent CBGTC projectome evidence, the need to study other pathways has become increasingly clear. In this review, we examine the current status of our knowledge of morphological and electrophysiological alterations of those pathways in animal models of HD. Based on recent studies, there is accumulating evidence that synaptic disconnection, particularly along excitatory pathways, is pervasive and almost universal in HD, thus supporting a critical role of the huntingtin protein in synaptic transmission.
Keywords: Basal ganglia; Disconnection; Genetic models; Huntington's disease; Synaptic activity.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Albin RL, Reiner A, Anderson KD, Dure LS, Handelin B, et al., 1992. Preferential loss of striato-external pallidal projection neurons in presymptomatic Huntington’s disease. Ann. Neurol 31, 425–430. - PubMed
-
- Alexander GE, DeLong MR, Strick PL, 1986. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu. Rev. Neurosci 9, 357–381. - PubMed

