Microfluidic characterisation reveals broad range of SARS-CoV-2 antibody affinity in human plasma
- PMID: 34848436
- PMCID: PMC8645332
- DOI: 10.26508/lsa.202101270
Microfluidic characterisation reveals broad range of SARS-CoV-2 antibody affinity in human plasma
Abstract
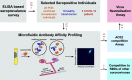
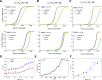
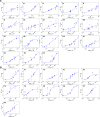
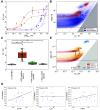
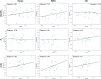
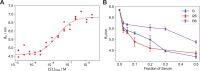
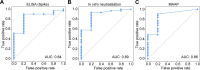
The clinical outcome of SARS-CoV-2 infections, which can range from asymptomatic to lethal, is crucially shaped by the concentration of antiviral antibodies and by their affinity to their targets. However, the affinity of polyclonal antibody responses in plasma is difficult to measure. Here we used microfluidic antibody affinity profiling (MAAP) to determine the aggregate affinities and concentrations of anti-SARS-CoV-2 antibodies in plasma samples of 42 seropositive individuals, 19 of which were healthy donors, 20 displayed mild symptoms, and 3 were critically ill. We found that dissociation constants, K d, of anti-receptor-binding domain antibodies spanned 2.5 orders of magnitude from sub-nanomolar to 43 nM. Using MAAP we found that antibodies of seropositive individuals induced the dissociation of pre-formed spike-ACE2 receptor complexes, which indicates that MAAP can be adapted as a complementary receptor competition assay. By comparison with cytopathic effect-based neutralisation assays, we show that MAAP can reliably predict the cellular neutralisation ability of sera, which may be an important consideration when selecting the most effective samples for therapeutic plasmapheresis and tracking the success of vaccinations.
© 2021 Schneider et al.
Conflict of interest statement
TPJ Knowles is a member of the board of directors of Fluidic Analytics. A Aguzzi is a member of the board of directors of Mabylon AG which has funded antibody-related work in the Aguzzi lab in the past. V Denninger, S Fiedler, H Fiegler are employees of Fluidic Analytics, MM Schneider, CK Xu, G Meisl and V Kosmoliaptsis are consultants. All other authors declare no competing interests.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
