Patient and caregiver outcomes with levodopa-carbidopa intestinal gel in advanced Parkinson's disease
- PMID: 34848716
- PMCID: PMC8633325
- DOI: 10.1038/s41531-021-00246-y
Patient and caregiver outcomes with levodopa-carbidopa intestinal gel in advanced Parkinson's disease
Abstract
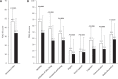
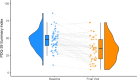
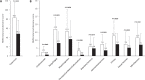
Levodopa-carbidopa intestinal gel (LCIG) has shown to be efficacious in motor and non-motor symptoms (NMS). Nevertheless, studies with patient Quality of Life (QoL) as a primary endpoint are scarce. To assess the effect of LCIG on Advanced Parkinson's Disease (APD) patients QoL. Secondarily, the impact on motor symptoms and NMS, emotional well-being, treatment satisfaction, and caregiver QoL, stress, disease burden, anxiety, depression, and work impairment were also investigated. In this prospective, 6-month multicenter postmarketing observational study, LCIG was administered to 59 patients with APD. Endpoints were assessed using validated scales and questionnaires. LCIG significantly improved patient QoL (PDQ-39 mean change ± standard deviation from baseline, -12.8 ± 14.6; P < 0.0001), motor symptoms (UPDRS-III in "On," -6.5 ± 11.8; P = 0.0002), NMS (NMSS, -35.7 ± 31.1; P < 0.0001), mood (Norris/Bond-Lader VAS, -6.6 ± 21.1; P = 0.0297), fatigue (PFS-16, -0.6 ± 1.0; P = 0.0003), depression (BDI-II, -5.1 ± 9.4; P = 0.0002), anxiety (BAI, -6.2 ± 9.6; P < 0.0001), and patient treatment satisfaction (SATMED-Q, 16.1 ± 16.8; P < 0.0001). There were significant correlations between the change from baseline to 6 months between PDQ-39 and UPDRS-IV, NMSS, BAI, BDI-II, AS, and PFS-16 scores, and Norris/Bond-Lader alertness/sedation factor. Caregiver anxiety also improved (Goldberg anxiety scale, -1.1 ± 1.0; P = 0.0234), but the clinical relevance of this finding is questionable. The serious adverse events reported were similar to those previously described for LCIG. In patients with APD, LCIG improves QoL, motor symptoms and NMS, emotional well-being, and satisfaction with the treatment. Improvement in patient QoL is associated with improvements in motor complications, NMS, anxiety, depression, apathy and fatigue. Improvements in patients' QoL does not correspond with improvements in caregivers' QoL or burden.
© 2021. The Author(s).
Conflict of interest statement
The author declares no competing interests. The disclosures of the authors are: F. Valldeoriola has received honoraria for speaking services and advisory boards from AbbVie, Zambon, Teva, Medtronic, and Boston Scientific. M.J. Catalán has received honoraria for consulting, advisory services, speaking services, and research from AbbVie Laboratories and Merz. F. Escamilla-Sevilla has received honoraria for advisory services, consulting, lecturing, support in assisting scientific meetings, and research from AbbVie, Italfarmaco, Medtronic, Merz, Teva, UCB Pharma, and Zambon. E. Freire has received honoraria from AbbVie, UCB, Zambon, Bial, and Eisai. J. Olivares has received honoraria in 2016 from AbbVie, UCB, Zambon, Teva, and Eisai. E. Cubo has received honoraria from AbbVie and Zambon. D. Santos Garcia has received honoraria from AbbVie, UCB Pharma, Teva, Zambon, KRKA, and Lundbeck. M. Calopa has received honoraria from AbbVie and Zambon. P. Martínez-Martín has received honoraria from National School of Public Health (ISCIII), Britannia, and Editorial Viguera for lecturing in courses; International Parkinson and Movement Disorder Society for management of the Program on Rating Scales; Bial, and Zambon for advice in clinical-epidemiological studies. Financial support by the International Parkinson and Movement Disorder Society for attending the IPMDS International Congress 2019. Grant for Research: International Parkinson and Movement Disorder Society, for development and validation of the MDS-NMS. J.C. Parra is an employee of AbbVie and holds AbbVie stock and/or stock options. G. Arroyo is an employee of AbbVie. J.M. Arbelo has received honoraria from AbbVie, Zambon, Bial, and Teva.
Figures

References
-
- Chapuis S, Ouchchane L, Metz O, Gerbaud L, Durif F. Impact of the motor complications of Parkinson’s disease on the quality of life. Mov. Disord. 2005;20:224–230. - PubMed
-
- Deuschl G, et al. A randomized trial of deep-brain stimulation for Parkinson’s disease. N. Engl. J. Med. 2006;355:896–908. - PubMed
-
- Katzenschlager R, et al. Apomorphine subcutaneous infusion in patients with Parkinson’s disease with persistent motor fluctuations (TOLEDO): a multicentre, double-blind, randomised, placebo-controlled trial. Lancet Neurol. 2018;17:749–759. - PubMed
-
- Peto V, Jenkinson C, Fitzpatrick R, Greenhall R. The development and validation of a short measure of functioning and well being for individuals with Parkinson’s disease. Qual. Life. Res. 1995;4:241–248. - PubMed

