3D in vitro M2 macrophage model to mimic modulation of tissue repair
- PMID: 34848722
- PMCID: PMC8633361
- DOI: 10.1038/s41536-021-00193-5
3D in vitro M2 macrophage model to mimic modulation of tissue repair
Abstract
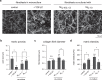
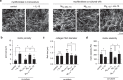
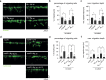
Distinct anti-inflammatory macrophage (M2) subtypes, namely M2a and M2c, are reported to modulate the tissue repair process tightly and chronologically by modulating fibroblast differentiation state and functions. To establish a well-defined three-dimensional (3D) cell culture model to mimic the tissue repair process, we utilized THP-1 human monocytic cells and a 3D collagen matrix as a biomimetic tissue model. THP-1 cells were differentiated into macrophages, and activated using IL-4/IL-13 (MIL-4/IL-13) and IL-10 (MIL-10). Both activated macrophages were characterized by both their cell surface marker expression and cytokine secretion profile. Our cell characterization suggested that MIL-4/IL-13 and MIL-10 demonstrate M2a- and M2c-like subtypes, respectively. To mimic the initial and resolution phases during the tissue repair, both activated macrophages were co-cultured with fibroblasts and myofibroblasts. We showed that MIL-4/IL-13 were able to promote matrix synthesis and remodeling by induction of myofibroblast differentiation via transforming growth factor beta-1 (TGF-β1). On the contrary, MIL-10 demonstrated the ability to resolve the tissue repair process by dedifferentiation of myofibroblast via IL-10 secretion. Overall, our study demonstrated the importance and the exact roles of M2a and M2c-like macrophage subtypes in coordinating tissue repair in a biomimetic model. The established model can be applied for high-throughput platforms for improving tissue healing and anti-fibrotic drugs testing, as well as other biomedical studies.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
Grants and funding
LinkOut - more resources
Full Text Sources

