Meteorin-β/Meteorin like/IL-41 attenuates airway inflammation in house dust mite-induced allergic asthma
- PMID: 34848868
- PMCID: PMC8803866
- DOI: 10.1038/s41423-021-00803-8
Meteorin-β/Meteorin like/IL-41 attenuates airway inflammation in house dust mite-induced allergic asthma
Abstract
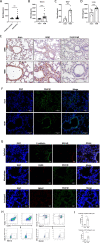
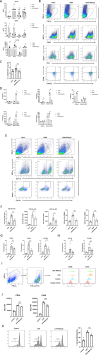
We sought to examine the regulatory effect of Meteorin-β (Metrnβ)/Meteorin like (Metrnl)/IL-41 on lung inflammation in allergic asthma. We found that Metrnβ was elevated significantly in asthmatic patients and in mice with allergic asthma induced by house dust mite (HDM) extract. Upon exposure to HDM, Metrnβ was secreted predominantly by airway epithelial cells and inflammatory cells, including macrophages and eosinophils. The increased Metrnβ effectively blocked the development of airway hyperreactivity (AHR) and decreased inflammatory cell airway infiltration and type 2 cytokine production, which was associated with downregulated DC-mediated adaptive immune responses. Moreover, Metrnβ impaired the maturation and function of bone marrow-derived dendritic cells in vitro. Asthmatic mice adoptively transferred with dendritic cells isolated from Metrnβ-treated allergic mice displayed decreased AHR, airway inflammation, and lung injury. Metrnβ also displayed anti-inflammatory properties in immunodeficient SCID mice with allergic asthma and in in vitro 3D ALI airway models. Moreover, blockade of Metrnβ by anti-Metrnβ antibody treatment promoted the development of allergic asthma. These results revealed the unappreciated protective roles of Metrnβ in alleviating DC-mediated Th2 inflammation in allergic asthma, providing the novel treatment strategy of therapeutic targeting of Metrnβ in allergic asthma.
Keywords: Air–liquid interface; Allergic asthma; Dendritic cells; Eosinophils; Metrnβ.
© 2021. The Author(s), under exclusive licence to CSI and USTC.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Schatz M, Rosenwasser L. The allergic asthma phenotype. J Allergy Clin Immunol Pract. 2014;2:645–8. - PubMed
-
- Christiansen SC, Zuraw BL. Treatment of hypertension in patients with asthma. N Engl J Med. 2019;381:1046–57. - PubMed
-
- Hekking PW, Wener RR, Amelink M, Zwinderman AH, Bouvy ML, Bel EH. The prevalence of severe refractory asthma. J Allergy Clin Immunol. 2015;135:896–902. - PubMed
-
- Mishra A, Yao X, Saxena A, Gordon EM, Kaler M, Cuento RA, et al. Low-density lipoprotein receptor-related protein 1 attenuates house dust mite-induced eosinophilic airway inflammation by suppressing dendritic cell-mediated adaptive immune responses. J Allergy Clin Immunol. 2018;142:1066–79.e1066. - PMC - PubMed

