Gut Microbiota and Sunitinib-Induced Diarrhea in Metastatic Renal Cell Carcinoma: A Pilot Study
- PMID: 34849023
- PMCID: PMC8612664
- DOI: 10.2147/CMAR.S328451
Gut Microbiota and Sunitinib-Induced Diarrhea in Metastatic Renal Cell Carcinoma: A Pilot Study
Abstract
Introduction: Sunitinib-induced diarrhea seriously affects the prognosis of patients with metastatic renal cell carcinoma (mRCC) and reduces their quality of life. We aim to explore and find the relationship between sunitinib-induced diarrhea and gut microbiota.
Methods: Feces were collected from 31 mRCC patients receiving sunitinib treatment. To characterize the feces gut microbiome profiles of patients, the V3-V4 region of 16S rRNA sequencing was carried out in this study.
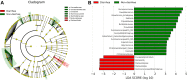
Results: Gut microbial diversity was decreased both in the severe diarrhea group and in the sunitinib-post group. The microbial composition with higher abundance of Bacteroides (mucus degrading bacteria) and lower abundance of Faecalibacterium, Oscillospira, Ruminococcaceae, Eubacterium and Coriobacteriaceae (butyrate-producing bacteria) were found in patients with diarrhea. Interestingly, the abundance of Actinobacteria was decreased in patients receiving sunitinib with severe diarrhea.
Conclusion: This study reported an association between gut microbiota and sunitinib-induced diarrhea. Defects of the butyrate-producing bacteria and the increase in Bacteroides may be the physiological basis of sunitinib-induced diarrhea.
Keywords: 16S rRNA sequencing; butyrate-producing bacteria; gut microbial diversity; sunitinib-induced diarrhea.
© 2021 Su et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures




References
-
- Sh Z. Adjuvant Sunitinib in Renal-Cell Carcinoma. N Engl J Med. 2017;376(9):893. - PubMed
LinkOut - more resources
Full Text Sources

