Relationship and improvement strategies between drug nanocarrier characteristics and hemocompatibility: What can we learn from the literature
- PMID: 34849162
- PMCID: PMC8609445
- DOI: 10.1016/j.ajps.2020.12.002
Relationship and improvement strategies between drug nanocarrier characteristics and hemocompatibility: What can we learn from the literature
Abstract
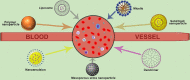
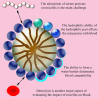
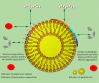
This article discusses the various blood interactions that may occur with various types of nano drug-loading systems. Nanoparticles enter the blood circulation as foreign objects. On the one hand, they may cause a series of inflammatory reactions and immune reactions, resulting in the rapid elimination of immune cells and the reticuloendothelial system, affecting their durability in the blood circulation. On the other hand, the premise of the drug-carrying system to play a therapeutic role depends on whether they cause coagulation and platelet activation, the absence of hemolysis and the elimination of immune cells. For different forms of nano drug-carrying systems, we can find the characteristics, elements and coping strategies of adverse blood reactions that we can find in previous researches. These adverse reactions may include destruction of blood cells, abnormal coagulation system, abnormal effects of plasma proteins, abnormal blood cell behavior, adverse immune and inflammatory reactions, and excessive vascular stimulation. In order to provide help for future research and formulation work on the blood compatibility of nano drug carriers.
Keywords: Adverse interaction; Hemocompatibility; Improvement strategy; Nano-drug delivery system.
© 2021 Shenyang Pharmaceutical University. Published by Elsevier B.V.
Figures





References
-
- Bender E.A., Adorne M.D., Colome L.M., Abdalla D.S.P., Guterres S.S., Pohlmann A.R. Hemocompatibility of poly(varepsilon-caprolactone) lipid-core nanocapsules stabilized with polysorbate 80-lecithin and uncoated or coated with chitosan. Int J Pharm. 2012;426(1–2):271–279. - PubMed
-
- Kumar Y., Kuche K., Swami R., Katiyar S.S., Chaudhari D., Katare P.B., et al. Exploring the potential of novel pH sensitive lipoplexes for tumor targeted gene delivery with reduced toxicity. Int J Pharm. 2020;573 - PubMed
-
- Ali S., Amin M.U., Ali M.Y., Tariq I., Pinnapireddy S.R., Duse L., et al. Wavelength dependent photo-cytotoxicity to ovarian carcinoma cells using temoporfin loaded tetraether liposomes as efficient drug delivery system. Eur J Pharm Biopharm. 2020;150:50–65. - PubMed
-
- Amin K., Dannenfelser R.M. In vitro hemolysis: guidance for the pharmaceutical scientist. J Pharm Sci. 2006;95(6):1173–1176. - PubMed
-
- Mittal P., Vardhan H., Ajmal G., Bonde G.V., Kapoor R., Mittal A., et al. Formulation, optimization, hemocompatibility and pharmacokinetic evaluation of PLGA nanoparticles containing paclitaxel. Drug Dev Ind Pharm. 2019;45(3):365–378. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

