Discovering New Chemistry with an Autonomous Robotic Platform Driven by a Reactivity-Seeking Neural Network
- PMID: 34849401
- PMCID: PMC8620554
- DOI: 10.1021/acscentsci.1c00435
Discovering New Chemistry with an Autonomous Robotic Platform Driven by a Reactivity-Seeking Neural Network
Abstract
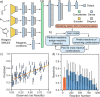
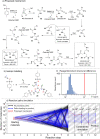
We present a robotic chemical discovery system capable of navigating a chemical space based on a learned general association between molecular structures and reactivity, while incorporating a neural network model that can process data from online analytics and assess reactivity without knowing the identity of the reagents. Working in conjunction with this learned knowledge, our robotic platform is able to autonomously explore a large number of potential reactions and assess the reactivity of mixtures, including unknown chemical spaces, regardless of the identity of the starting materials. Through the system, we identified a range of chemical reactions and products, some of which were well-known, some new but predictable from known pathways, and some unpredictable reactions that yielded new molecules. The validation of the system was done within a budget of 15 inputs combined in 1018 reactions, further analysis of which allowed us to discover not only a new photochemical reaction but also a new reactivity mode for a well-known reagent (p-toluenesulfonylmethyl isocyanide, TosMIC). This involved the reaction of 6 equiv of TosMIC in a "multistep, single-substrate" cascade reaction yielding a trimeric product in high yield (47% unoptimized) with the formation of five new C-C bonds involving sp-sp2 and sp-sp3 carbon centers. An analysis reveals that this transformation is intrinsically unpredictable, demonstrating the possibility of a reactivity-first robotic discovery of unknown reaction methodologies without requiring human input.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Reymond J. L.; Ruddigkeit L.; Blum L.; van Deursen R. The enumeration of chemical space. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 717–733. 10.1002/wcms.1104. - DOI
-
- Schwaller P.; Probst D.; Vaucher A. C.; et al. Mapping the space of chemical reactions using attention-based neural networks. Nat. Mach Intell 2021, 3, 144–152. 10.1038/s42256-020-00284-w. - DOI
-
- Herges R. Reaction planning: prediction of new organic reactions. J. Chem. Inf. Comput. Sci. 1990, 30, 377–383. 10.1021/ci00068a006. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

