Wide-field calcium imaging of cortex-wide activity in awake, head-fixed mice
- PMID: 34849490
- PMCID: PMC8608657
- DOI: 10.1016/j.xpro.2021.100973
Wide-field calcium imaging of cortex-wide activity in awake, head-fixed mice
Abstract
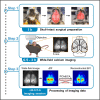
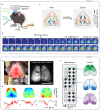
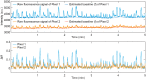
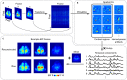
Characterizing cortex-wide neural activity is essential for understanding large-scale interactions among distributed cortical regions. Here, we describe a protocol using wide-field calcium imaging to monitor the cortex-wide activity in awake, head-fixed mice. This approach provides sufficient signal-to-noise ratio and spatiotemporal resolution to capture large-scale neural activity in behaving mice on a moment-by-moment basis. The use of genetically encoded calcium indicators allows longitudinal imaging over months and can achieve cell-type specificity. We also describe a pipeline to process the imaging data. For complete details on the use and execution of this protocol, please refer to Makino et al. (2017) and Liu et al. (2021).
© 2021 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

