Real-world effectiveness of the mRNA-1273 vaccine against COVID-19: Interim results from a prospective observational cohort study
- PMID: 34849505
- PMCID: PMC8614600
- DOI: 10.1016/j.lana.2021.100134
Real-world effectiveness of the mRNA-1273 vaccine against COVID-19: Interim results from a prospective observational cohort study
Abstract
Background: Phase 3 trials found mRNA-1273 was highly effective in preventing COVID-19. We conducted a prospective cohort study at Kaiser Permanente Southern California (KPSC) to determine the real-world vaccine effectiveness (VE) of mRNA-1273 in preventing COVID-19 infection and severe disease.
Methods: For this planned interim analysis, individuals aged ≥18 years receiving 2 doses of mRNA-1273 ≥24 days apart (18/12/2020-31/03/2021) were 1:1 matched to randomly selected unvaccinated individuals by age, sex, and race/ethnicity, with follow-up through 30/06/2021. Outcomes were COVID-19 infection (SARS-CoV-2 positive molecular test or COVID-19 diagnosis code) or severe disease (COVID-19 hospitalization and COVID-19 hospital death). Adjusted hazard ratios (aHR) and confidence intervals (CI) for COVID-19 outcomes comparing vaccinated and unvaccinated individuals were estimated by Cox proportional hazards models accounting for multiple comparisons. Adjusted VE was calculated as (1-aHR)x100. Whole genome sequencing was performed on SARS-CoV-2 positive specimens from the KPSC population.
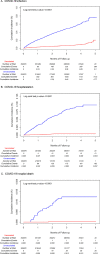
Findings: This analysis included 352,878 recipients of 2 doses of mRNA-1273 matched to 352,878 unvaccinated individuals. VE (99·3% CI) against COVID-19 infection was 87·4% (84·8-89·6%). VE against COVID-19 hospitalization and hospital death was 95·8% (90·7-98·1%) and 97·9% (66·9-99·9%), respectively. VE was higher against symptomatic (88·3% [98·3% CI: 86·1-90·2%]) than asymptomatic COVID-19 (72·7% [53·4-84·0%]), but was generally similar across age, sex, and racial/ethnic subgroups. VE among individuals with history of COVID-19 ranged from 8·2-33·6%. The most prevalent variants were Alpha (41·6%), Epsilon (17·5%), Delta (11·5%), and Gamma (9·1%), with Delta increasing to 54·0% of variants by June 2021.
Interpretation: These interim results provide reassuring evidence of the VE of 2 doses of mRNA-1273 across age, sex, and racial/ethnic subgroups, and against asymptomatic and symptomatic COVID-19, and severe COVID-19 outcomes. Among individuals with history of COVID-19, mRNA-1273 vaccination may offer added protection beyond immunity acquired from prior infection. Longer follow-up is needed to fully evaluate VE of mRNA-1273 against emerging SARS-CoV-2 variants.
Funding: Moderna Inc.
Keywords: COVID-19; Matched cohort; SARS-CoV-2; Vaccine effectiveness; Variants; mRNA-1273.
© 2021 Kaiser Permanente Southern California.
Conflict of interest statement
KJB was employed by Kaiser Permanente Southern California during the conduct of the study and is now an adjunct investigator at Kaiser Permanente Southern California. LSS, LQ, BKA, YL, GSL, YT, AF, HST, JET, HFT are employees of Kaiser Permanente Southern California, which has been contracted by Moderna for the conduct of this present study. CAT is an employee of and a shareholder in Moderna Inc. KJB received funding from GlaxoSmithKline, Dynavax, Pfizer, Gilead, and Seqirus unrelated to this manuscript. LSS received funding from GlaxoSmithKline, Dynavax, and Seqirus unrelated to this manuscript. LQ received funding from GlaxoSmithKline and Dynavax unrelated to this manuscript. BKA received funding from GlaxoSmithKline, Dynavax, Seqirus and Pfizer unrelated to this manuscript. YL received funding from GlaxoSmithKline, Seqirus and Pfizer unrelated to this manuscript. GSL received funding from GlaxoSmithKline unrelated to this manuscript. YT received funding from GlaxoSmithKline unrelated to this manuscript. AF received funding from Pfizer, GlaxoSmithKline, and Gilead unrelated to this manuscript. HST received funding from GlaxoSmithKline, Pfizer, ALK, and Wellcome unrelated to this manuscript. JET received funding from Pfizer unrelated to this manuscript. HFT received funding from GlaxoSmithKline and Seqirus unrelated to this manuscript; HFT also served in advisory boards for Janssen and Pfizer.
Figures

References
-
- United States Food and Drug Administration. Moderna COVID-19 Vaccine. December 18 2020. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-dise... Accessed July 19 2021.
-
- United States Food and Drug Administration. Pfizer-BioNTech COVID-19 Vaccine. December 11 2020. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-dise.... Accessed July 19 2021.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

