Effect of maternal prenatal and postpartum vitamin D supplementation on offspring bone mass and muscle strength in early childhood: follow-up of a randomized controlled trial
- PMID: 34849536
- PMCID: PMC8895216
- DOI: 10.1093/ajcn/nqab396
Effect of maternal prenatal and postpartum vitamin D supplementation on offspring bone mass and muscle strength in early childhood: follow-up of a randomized controlled trial
Erratum in
-
Corrigendum to "Effect of maternal prenatal and postpartum vitamin D supplementation on offspring bone mass and muscle strength in early childhood: follow-up of a randomized controlled trial [The American Journal of Clinical Nutrition, Volume 115, Issue 3, March 2022, Pages 770-780]".Am J Clin Nutr. 2023 May;117(5):1047. doi: 10.1016/j.ajcnut.2023.03.004. Am J Clin Nutr. 2023. PMID: 37137609 Free PMC article. No abstract available.
Abstract
Background: Maternal vitamin D status during pregnancy and lactation is a modifiable factor that may influence offspring musculoskeletal outcomes. However, few randomized trials have tested the effects of prenatal or postpartum vitamin D supplementation on offspring bone and muscle development.
Objectives: The aim was to examine hypothesized effects of improvements in early-life vitamin D status on childhood musculoskeletal health in Dhaka, Bangladesh.
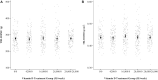
Methods: In a previously completed, double-blind, dose-ranging trial, healthy pregnant women (n = 1300) were recruited at 17-24 weeks' gestation and randomly assigned to a prenatal/postpartum regimen of 0/0, 4200/0, 16,800/0, 28,000/0, or 28,000/28,000 IU cholecalciferol (vitamin D3)/wk until 26 wk postpartum. In this new report, we describe additional follow-up at 4 y of age (n = 642) for longer-term outcomes. Bone mineral content (BMC) and areal bone mineral density (aBMD) were measured by DXA. Grip strength was tested using a hand-held dynamometer. The primary comparison was children of women assigned to 28,000 IU/wk prenatally compared with placebo. Differences are expressed as means and 95% CIs.
Results: Total-body-less-head (TBLH) BMC, TBLH aBMD, and grip strength were similar in the combined high-dose prenatal (28,000/0 and 28,000/28,000 IU/wk) compared with placebo groups (mean difference [95% CI] = 0.61 g [-10.90, 12.13], 0.0004 g/cm2 [-0.0089, 0.0097], and 0.02 kg [-0.26, 0.31], respectively). In dose-ranging analyses, TBLH BMC and aBMD, whole-body BMC and aBMD, and grip strength in each of the prenatal vitamin D groups were not significantly different from placebo (P > 0.05 for all comparisons). Only head aBMD was greater in children of women assigned to the 28,000/28,000-IU regimen compared with placebo (mean difference [95% CI] = 0.024 g/cm2 [0.0009, 0.047], P = 0.042); the effect was attenuated upon adjustment for child height, weight, and sex (P = 0.11).
Conclusions: Maternal prenatal, with or without postpartum, vitamin D supplementation does not improve child BMC, aBMD, or grip strength at 4 y of age. The MDIG trial and present follow-up study were registered prospectively at www.clinicaltrials.gov as NCT01924013 and NCT03537443, respectively.
Keywords: areal bone mineral density; bone mineral content; grip strength; randomized controlled trial; vitamin D.
© The Author(s) 2021. Published by Oxford University Press on behalf of the American Society for Nutrition.
Figures
References
-
- Johnell O, Kanis JA, Oden A, Johansson H, De Laet C, Delmas P, Eisman JA, Fujiwara S, Kroger H, Mellstrom Det al. Predictive value of BMD for hip and other fractures. J Bone Miner Res. 2005;20(7):1185–94. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

