Six-dimensional dynamic tractography atlas of language connectivity in the developing brain
- PMID: 34849596
- PMCID: PMC8677551
- DOI: 10.1093/brain/awab225
Six-dimensional dynamic tractography atlas of language connectivity in the developing brain
Abstract
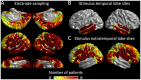
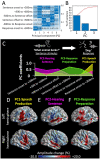
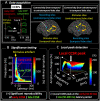
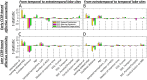
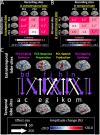
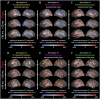
During a verbal conversation, our brain moves through a series of complex linguistic processing stages: sound decoding, semantic comprehension, retrieval of semantically coherent words, and overt production of speech outputs. Each process is thought to be supported by a network consisting of local and long-range connections bridging between major cortical areas. Both temporal and extratemporal lobe regions have functional compartments responsible for distinct language domains, including the perception and production of phonological and semantic components. This study provides quantitative evidence of how directly connected inter-lobar neocortical networks support distinct stages of linguistic processing across brain development. Novel six-dimensional tractography was used to intuitively visualize the strength and temporal dynamics of direct inter-lobar effective connectivity between cortical areas activated during each linguistic processing stage. We analysed 3401 non-epileptic intracranial electrode sites from 37 children with focal epilepsy (aged 5-20 years) who underwent extra-operative electrocorticography recording. Principal component analysis of auditory naming-related high-gamma modulations determined the relative involvement of each cortical area during each linguistic processing stage. To quantify direct effective connectivity, we delivered single-pulse electrical stimulation to 488 temporal and 1581 extratemporal lobe sites and measured the early cortico-cortical spectral responses at distant electrodes. Mixed model analyses determined the effects of naming-related high-gamma co-augmentation between connecting regions, age, and cerebral hemisphere on the strength of effective connectivity independent of epilepsy-related factors. Direct effective connectivity was strongest between extratemporal and temporal lobe site pairs, which were simultaneously activated between sentence offset and verbal response onset (i.e. response preparation period); this connectivity was approximately twice more robust than that with temporal lobe sites activated during stimulus listening or overt response. Conversely, extratemporal lobe sites activated during overt response were equally connected with temporal lobe language sites. Older age was associated with increased strength of inter-lobar effective connectivity especially between those activated during response preparation. The arcuate fasciculus supported approximately two-thirds of the direct effective connectivity pathways from temporal to extratemporal auditory language-related areas but only up to half of those in the opposite direction. The uncinate fasciculus consisted of <2% of those in the temporal-to-extratemporal direction and up to 6% of those in the opposite direction. We, for the first time, provided an atlas which quantifies and animates the strength, dynamics, and direction specificity of inter-lobar neural communications between language areas via the white matter pathways. Language-related effective connectivity may be strengthened in an age-dependent manner even after the age of 5.
Keywords: cortico-cortical evoked potentials; dynamic DWI tractography; high-gamma activity; intracranial electroencephalography; paediatric epilepsy surgery.
© The Author(s) (2021). Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures

References
-
- Hamberger MJ, Seidel WT, Mckhann GM, Perrine K, Goodman RR.. Brain stimulation reveals critical auditory naming cortex. Brain. 2005;128(Pt 11):2742–2749. - PubMed
-
- Hickok G, Poeppel D.. The cortical organization of speech processing. Nat Rev Neurosci. 2007;8(5):393–402. - PubMed
-
- Chang EF, Raygor KP, Berger MS.. Contemporary model of language organization: An overview for neurosurgeons. J Neurosurg. 2015;122(2):250–261. - PubMed

