Cycloserine and Linezolid for Tuberculosis Meningitis: Pharmacokinetic Evidence of Potential Usefulness
- PMID: 34849645
- PMCID: PMC9464073
- DOI: 10.1093/cid/ciab992
Cycloserine and Linezolid for Tuberculosis Meningitis: Pharmacokinetic Evidence of Potential Usefulness
Abstract
Background: The ability of antituberculosis drugs to cross the blood-brain barrier and reach the central nervous system is critical to their effectiveness in treating tuberculosis meningitis (TBM). We sought to fill a critical knowledge gap by providing data on the ability of new and repurposed antituberculosis drugs to penetrate into the cerebrospinal fluid (CSF).
Methods: We conducted a clinical pharmacology study among patients treated for TBM in Tbilisi, Georgia, from January 2019 until January 2020. Serial serum and CSF samples were collected while patients were hospitalized. CSF was collected from routine lumbar punctures with the timing of the lumbar puncture alternating between 2 and 6 hours to capture early and late CSF penetration.
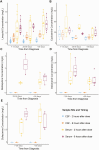
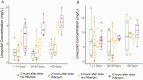
Results: A total of 17 patients treated for TBM (8 with confirmed disease) were included; all received linezolid, with a subset receiving cycloserine (5), clofazimine (5), delamanid (4), and bedaquiline (2). All CSF measurements of bedaquiline (12), clofazimine (24), and delamanid (19) were below the limit of detection. The median CSF concentrations of cycloserine at 2 and 6 hours were 15.90 and 15.10 µg/mL with adjusted CSF/serum ratios of 0.52 and 0.66. CSF concentrations of linezolid were 0.90 and 3.14 µg/mL at 2 and 6 hours, with adjusted CSF/serum ratios of 0.25 and 0.59, respectively. CSF serum linezolid concentrations were not affected by rifampin coadministration.
Conclusions: Based on moderate to high CSF penetration, linezolid and cycloserine may be effective drugs for TBM treatment, whereas the utility of bedaquiline, delamanid, and clofazimine is uncertain given their low CSF penetration.
Keywords: cerebrospinal fluid; meningitis; pharmacokinetics; pharmacology; tuberculosis.
© The Author(s) 2021. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Conflict of interest statement
Potential conflicts of interest. J. M. C. reports grants from the NIH and contract from the Centers for Disease Control and Prevention outside of the conduct of the study. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures
Comment in
-
Cerebrospinal Fluid and Tuberculous Meningitis.Clin Infect Dis. 2023 Jul 5;77(1):158. doi: 10.1093/cid/ciad186. Clin Infect Dis. 2023. PMID: 36987607 Free PMC article. No abstract available.
References
-
- Wilkinson RJ, Rohlwink U, Misra UK, et al. Tuberculous meningitis. Nat Rev Neurol 2017; 13:581–98. - PubMed
-
- Donald PR. Cerebrospinal fluid concentrations of antituberculosis agents in adults and children. Tuberculosis (Edinb) 2010; 90:279–92. - PubMed
-
- Thwaites GE, Lan NT, Dung NH, et al. Effect of antituberculosis drug resistance on response to treatment and outcome in adults with tuberculous meningitis. J Infect Dis 2005; 192:79–88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

