Randomized Trial of Metformin With Anti-Tuberculosis Drugs for Early Sputum Conversion in Adults With Pulmonary Tuberculosis
- PMID: 34849651
- PMCID: PMC9427151
- DOI: 10.1093/cid/ciab964
Randomized Trial of Metformin With Anti-Tuberculosis Drugs for Early Sputum Conversion in Adults With Pulmonary Tuberculosis
Abstract
Background: Metformin, by reducing intracellular Mycobacterium tuberculosis growth, can be considered an adjunctive therapy to anti-tuberculosis treatment (ATT). We determined whether metformin with standard ATT reduces time to sputum culture conversion and tissue inflammation in adults with pulmonary tuberculosis (PTB).
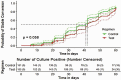
Methods: In a randomized, 8-week, clinical trial, newly diagnosed, culture-positive PTB patients were randomized to standard ATT (HREZ = control arm) or standard ATT plus daily 1000 mg metformin (MET-HREZ = Metformin with Rifampicin [METRIF] arm) for 8 weeks during 2018-2020 at 5 sites in India. The primary end point was time to sputum culture conversion by liquid culture during 8 weeks of ATT. Plasma inflammatory markers were estimated in a subset. A Cox proportional hazard model was used to estimate time and predictors of culture conversion.
Results: Of the 322 patients randomized, 239 (74%) were male, and 212 (66%) had bilateral disease on chest radiograph with 54 (18%) showing cavitation. The median time to sputum culture conversion by liquid culture was 42 days in the METRIF arm and 41 days in the control arm (hazard ratio, 0.8; 95% confidence interval [CI], .624-1.019). After 8 weeks of ATT, cavitary lesions on X-ray (7, 5.3% vs 18, 12.9%; relative risk, 0.42; 95% CI, .18-.96; P = .041) and inflammatory markers were significantly lower in the METRIF arm. Higher body mass index and lower sputum smear grading were associated with faster sputum culture conversion.
Conclusions: The addition of metformin to standard ATT did not hasten sputum culture conversion but diminished excess inflammation, thus reducing lung tissue damage as seen by faster clearance on X-ray and reduced inflammatory markers.
Clinical trials registration: Clinical Trial Registry of India (CTRI/2018/01/011176).
Keywords: host-directed therapy; immunomodulation; metformin; pharmacokinetics; tuberculosis.
© The Author(s) 2021. Published by Oxford University Press for the Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Figures



References
-
- World Health Organization . Guidelines for treatment of drug-susceptible tuberculosis and patient care, 2017 update. Geneva, Switzerland: World Health Organization; 2017.
-
- Raviglione MC, Ditiu L.. Setting new targets in the fight against tuberculosis. Nat Med 2013; 19:263. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

