A Higher Antibody Response Is Generated With a 6- to 7-Week (vs Standard) Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Vaccine Dosing Interval
- PMID: 34849655
- PMCID: PMC8690265
- DOI: 10.1093/cid/ciab938
A Higher Antibody Response Is Generated With a 6- to 7-Week (vs Standard) Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Vaccine Dosing Interval
Abstract
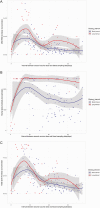
The optimal dosing interval for severe acute respiratory syndrome coronavirus 2 vaccines remains controversial. In this prospective study, we compared serology results of paramedics vaccinated with mRNA vaccines at the recommended short (17-28 days) vs long (42-49 days) interval. We found that a long dosing interval resulted in higher spike, receptor binding domain, and spike N terminal domain antibody concentrations.
Keywords: COVID-19; SARS-CoV-2; antibodies; spike; vaccine.
© The Author(s) 2021. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

