Molecular Derangements and the Diagnosis of ACTH-Dependent Cushing's Syndrome
- PMID: 34849663
- PMCID: PMC9512149
- DOI: 10.1210/endrev/bnab046
Molecular Derangements and the Diagnosis of ACTH-Dependent Cushing's Syndrome
Abstract
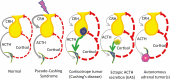
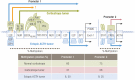
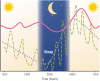
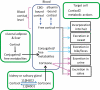
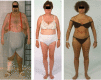
Endogenous Cushing's syndrome (CS) is associated with morbidities (diabetes, hypertension, clotting disorders) and shortens life because of infections, pulmonary thromboembolism, and cardiovascular disease. Its clinical presentation is immensely variable, and diagnosis and treatment are often delayed. Thus, there are many opportunities for basic and clinical research leading to better tests, faster diagnosis, and optimized medical treatments. This review focuses on CS caused by excessive adrenocorticotropin (ACTH) production. It describes current concepts of the regulation of ACTH synthesis and secretion by normal corticotropes and mechanisms by which dysregulation occurs in corticotrope (termed "Cushing's disease") and noncorticotrope (so-called ectopic) ACTH-producing tumors. ACTH causes adrenal gland synthesis and pulsatile release of cortisol; the excess ACTH in these forms of CS leads to the hypercortisolism of endogenous CS. Again, the differences between healthy individuals and those with CS are highlighted. The clinical presentations and their use in the interpretation of CS screening tests are described. The tests used for screening and differential diagnosis of CS are presented, along with their relationship to cortisol dynamics, pathophysiology, and negative glucocorticoid feedback regulation in the two forms of ACTH-dependent CS. Finally, several gaps in current understanding are highlighted in the hope of stimulating additional research into this challenging disorder.
Published by Oxford University Press on behalf of the Endocrine Society 2021.
Figures

References
-
- Rubinstein G, Osswald A, Hoster E, et al. . Time to diagnosis in Cushing’s syndrome: a meta-analysis based on 5367 patients. J Clin Endocrinol Metab. 2020;105(3):e12-e23. - PubMed
-
- Upton AC, Furth J. Spontaneous and radiation-induced pituitary adenomas of mice. J Natl Cancer Inst. 1955;15(4):1005-1021. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

