Development of a patient decision aid for COVID-19 vaccination with the Comirnaty vaccine
- PMID: 34849748
- PMCID: PMC8690143
- DOI: 10.1093/fampra/cmab156
Development of a patient decision aid for COVID-19 vaccination with the Comirnaty vaccine
Abstract
Background: SARS-CoV-2 has been responsible for a pandemic since the beginning of 2020. Vaccine arrival brings a concrete solution to fight the virus. However, vaccine hesitancy is high. In France, the first available vaccine was Comirnaty from Pfizer-BioNTech. Shared decision-making, based on tools such as patient decision aids (PtDAs), can help patients make an informed choice about vaccination with Comirnaty.
Objective: The French College of Teachers in General Practice (CNGE) aimed to create a PtDA for people who have to decide whether they will receive the Comirnaty vaccine.
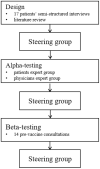
Methods: Development of the PtDA was performed according to the International Patient Decision Aids Standards (IPDAS). The initial design was based on a literature review and semistructured interviews with 17 patients to explore and clarify patients' expectations. A first draft of the PtDA was then alpha tested by a patient expert group and a physician expert group. The PtDA was finally beta tested in 14 prevaccine consultations. A steering group was consulted throughout the work. Patient support, community groups and the French National Authority for Health (HAS) were involved in the development process.
Results: A literature review identified one randomized trial on Comirnaty efficacy and safety. The first part of the PtDA allows patients to identify their own risk factors. The second part of the PtDA provides information on vaccination: benefits and risks, unknown data, and technical explanations about the mRNA vaccine.
Conclusions: We developed a PtDA to be used in primary care settings for shared decision-making regarding vaccination with Comirnaty.
Keywords: consumer health informatics; health information; health literacy; patient adherence; primary care; public health.
© The Author(s) 2021. Published by Oxford University Press. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
References
-
- Coronavirus could cost the global economy $2.7 trillion. Here’s how. Bloomberg.com. [accessed 2021 Feb 16]. https://www.bloomberg.com/graphics/2020-coronavirus-pandemic-global-econ....
-
- OECD Economic Outlook, December 2020. OECD. 2020. [accessed 2021 Feb 25]. https://www.oecd.org/economic-outlook/december-2020.
-
- IDSA guidelines on the treatment and management of patients with COVID-19. [accessed 2021 Feb 16]. https://www.idsociety.org/practice-guideline/covid-19-guideline-treatmen....
-
- EMA recommends first COVID-19 vaccine for authorisation in the EU. European Medicines Agency. 2020. [accessed 2021 Feb 25]. https://www.ema.europa.eu/en/news/ema-recommends-first-covid-19-vaccine-....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous