Vitamin A deficiency affects gene expression in the Drosophila melanogaster head
- PMID: 34849795
- PMCID: PMC8527478
- DOI: 10.1093/g3journal/jkab297
Vitamin A deficiency affects gene expression in the Drosophila melanogaster head
Abstract
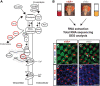
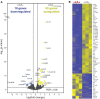
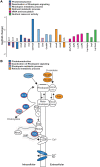
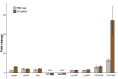
Insufficient dietary intake of vitamin A causes various human diseases. For instance, chronic vitamin A deprivation causes blindness, slow growth, impaired immunity, and an increased risk of mortality in children. In contrast to these diverse effects of vitamin A deficiency (VAD) in mammals, chronic VAD in flies neither causes obvious developmental defects nor lethality. As in mammals, VAD in flies severely affects the visual system: it impairs the synthesis of the retinal chromophore, disrupts the formation of the visual pigments (Rhodopsins), and damages the photoreceptors. However, the molecular mechanisms that respond to VAD remain poorly understood. To identify genes and signaling pathways that are affected by VAD, we performed RNA-sequencing and differential gene expression analysis in Drosophila melanogaster. We found an upregulation of genes that are essential for the synthesis of the retinal chromophore, specific aminoacyl-tRNA synthetases, and major nutrient reservoir proteins. We also discovered that VAD affects several genes that are required for the termination of the light response: for instance, we found a downregulation of both arrestin genes that are essential for the inactivation of Rhodopsin. A comparison of the VAD-responsive genes with previously identified blue light stress-responsive genes revealed that the two types of environmental stress trigger largely nonoverlapping transcriptome responses. Yet, both stresses increase the expression of seven genes with poorly understood functions. Taken together, our transcriptome analysis offers insights into the molecular mechanisms that respond to environmental stresses.
Keywords: Drosophila; carotene; chromophore; photoreceptor; phototransduction; retinoic acid; rhabdomere; rhodopsin; transcriptome; vision; visual pigment; vitamin A.
© The Author(s) 2021. Published by Oxford University Press on behalf of Genetics Society of America.
Figures

References
-
- Ahmad ST, Joyce MV, Boggess B, O'Tousa JE.. 2006. The role of drosophila ninaG oxidoreductase in visual pigment chromophore biogenesis. J Biol Chem. 281:9205–9209. - PubMed
-
- Bahner M, Frechter S, Da Silva N, Minke B, Paulsen R, et al. 2002. Light-regulated subcellular translocation of Drosophila TRPL channels induces long-term adaptation and modifies the light-induced current. Neuron. 34:83–93. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
