An RNAi screen for genes that affect nuclear morphology in Caenorhabditis elegans reveals the involvement of unexpected processes
- PMID: 34849797
- PMCID: PMC8527477
- DOI: 10.1093/g3journal/jkab264
An RNAi screen for genes that affect nuclear morphology in Caenorhabditis elegans reveals the involvement of unexpected processes
Abstract
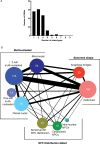
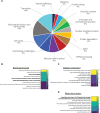
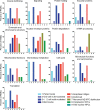
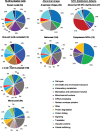
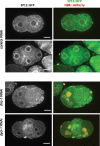
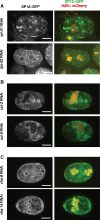
Aberration in nuclear morphology is one of the hallmarks of cellular transformation. However, the processes that, when mis-regulated, result aberrant nuclear morphology are poorly understood. In this study, we carried out a systematic, high-throughput RNAi screen for genes that affect nuclear morphology in Caenorhabditis elegans embryos. The screen employed over 1700 RNAi constructs against genes required for embryonic viability. Nuclei of early embryos are typically spherical, and their NPCs are evenly distributed. The screen was performed on early embryos expressing a fluorescently tagged component of the nuclear pore complex (NPC), allowing visualization of nuclear shape as well as the distribution of NPCs around the nuclear envelope. Our screen uncovered 182 genes whose downregulation resulted in one or more abnormal nuclear phenotypes, including multiple nuclei, micronuclei, abnormal nuclear shape, anaphase bridges, and abnormal NPC distribution. Many of these genes fall into common functional groups, including some that were not previously known to affect nuclear morphology, such as genes involved in mitochondrial function, the vacuolar ATPase, and the CCT chaperonin complex. The results of this screen add to our growing knowledge of processes that affect nuclear morphology and that may be altered in cancer cells that exhibit abnormal nuclear shape.
Keywords: C. elegans; CCT chaperonin; ER morphology; anaphase bridges; cytokinesis; micronuclei; nuclear envelope; paired nuclei; vacuolar ATPase.
Published by Oxford University Press 2021.
Figures

Similar articles
-
Sm protein down-regulation leads to defects in nuclear pore complex disassembly and distribution in C. elegans embryos.Dev Biol. 2012 May 15;365(2):445-57. doi: 10.1016/j.ydbio.2012.02.036. Epub 2012 Mar 8. Dev Biol. 2012. PMID: 22426005 Free PMC article.
-
Caenorhabditis elegans nucleoporins Nup93 and Nup205 determine the limit of nuclear pore complex size exclusion in vivo.Mol Biol Cell. 2003 Dec;14(12):5104-15. doi: 10.1091/mbc.e03-04-0237. Epub 2003 Aug 22. Mol Biol Cell. 2003. PMID: 12937276 Free PMC article.
-
Nuclear pore protein gp210 is essential for viability in HeLa cells and Caenorhabditis elegans.Mol Biol Cell. 2003 Oct;14(10):4230-7. doi: 10.1091/mbc.e03-04-0260. Epub 2003 Jul 11. Mol Biol Cell. 2003. PMID: 14517331 Free PMC article.
-
What can Caenorhabditis elegans tell us about the nuclear envelope?FEBS Lett. 2007 Jun 19;581(15):2794-801. doi: 10.1016/j.febslet.2007.03.052. Epub 2007 Mar 30. FEBS Lett. 2007. PMID: 17418822 Review.
-
From pore to kinetochore and back: regulating envelope assembly.Dev Cell. 2006 Sep;11(3):276-8. doi: 10.1016/j.devcel.2006.08.005. Dev Cell. 2006. PMID: 16950119 Review.
Cited by
-
Loss of the mitochondrial protein SPD-3 elevates PLK-1 levels and dysregulates mitotic events.Life Sci Alliance. 2023 Sep 8;6(11):e202302011. doi: 10.26508/lsa.202302011. Print 2023 Nov. Life Sci Alliance. 2023. PMID: 37684042 Free PMC article.
-
A multiparametric screen uncovers FDA-approved small molecules that potentiate the nuclear mechano-dysfunctions in ATR-defective cells.Sci Rep. 2024 Dec 28;14(1):30786. doi: 10.1038/s41598-024-80837-w. Sci Rep. 2024. PMID: 39730498 Free PMC article.
References
-
- Choi KY, Ji YJ, Dhakal BK, Yu JR, Cho C, et alet al.2003. Vacuolar-type H+-ATPase E subunit is required for embryogenesis and yolk transfer in Caenorhabditis elegans. Gene. 311:13–23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
