Identification of Mep1a as a susceptibility gene for atherosclerosis in mice
- PMID: 34849841
- PMCID: PMC8664597
- DOI: 10.1093/genetics/iyab160
Identification of Mep1a as a susceptibility gene for atherosclerosis in mice
Abstract
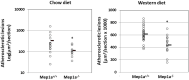
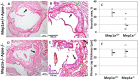
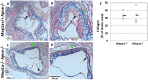
Atherosclerosis is the underlying cause of heart attack, ischemic stroke and peripheral arterial disease, and genetic factors involved remain mostly unidentified. We previously identified a significant locus on mouse chromosome 17 for atherosclerosis, Ath49, in an intercross between BALB/c and SM strains. Ath49 partially overlaps in the confidence interval with Ath22 mapped in an AKR × DBA/2 intercross. Bioinformatics analysis prioritized Mep1a, encoding meprin 1α metalloendopeptidase, as a likely candidate gene for Ath49. To prove causality, Mep1a-/-Apoe-/- mice were generated and compared with Mep1a+/+Apoe-/- mice for atherosclerosis development. Mep1a was found abundantly expressed in atherosclerotic lesions but not in healthy aorta and liver of mice. Mep1a-/- Apoe-/- mice exhibited significant reductions in both early and advanced lesion sizes. Loss of Mep1a led to decreased necrosis but increased macrophage and neutrophil contents in advanced lesions, reduced plasma levels of CXCL5 and an oxidative stress biomarker. In addition, Mep1a-/- mice had significantly reduced triglyceride levels on a chow diet. Thus, Mep1a is a susceptibility gene for atherosclerosis and aggravates atherosclerosis partially through action on oxidative stress and inflammation.
Keywords: Mep1a; Atherosclerosis; metalloendopeptidase; mice; oxidative stress.
© The Author(s) 2021. Published by Oxford University Press on behalf of Genetics Society of America. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures






References
-
- Arnold P, Otte A, Becker-Pauly C.. 2017. Meprin metalloproteases: molecular regulation and function in inflammation and fibrosis. Biochim Biophys Acta Mol Cell Res. 1864:2096–2104. doi:10.1016/j.bbamcr.2017.05.011. - PubMed
-
- Bankus JM, Bond JS.. 1996. Expression and distribution of meprin protease subunits in mouse intestine. Arch Biochem Biophys. 331:87–94. doi:10.1006/abbi.1996.0286. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

