Evaluation of the effect of sofosbuvir and daclatasvir in hospitalized COVID-19 patients: a randomized double-blind clinical trial (DISCOVER)
- PMID: 34849957
- PMCID: PMC8690191
- DOI: 10.1093/jac/dkab433
Evaluation of the effect of sofosbuvir and daclatasvir in hospitalized COVID-19 patients: a randomized double-blind clinical trial (DISCOVER)
Abstract
Background: The combination of sofosbuvir and daclatasvir has shown preliminary efficacy for hospitalized patients with COVID-19 in four open-label studies with small sample sizes. This larger trial aimed to assess if the addition of sofosbuvir/daclatasvir to standard care improved clinical outcomes in hospitalized patients with COVID-19.
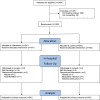
Methods: This was a placebo-controlled, double-blind, randomized clinical trial in adults hospitalized with COVID-19 at 19 hospitals in Iran. Patients were randomized to oral sofosbuvir/daclatasvir 400/60 mg once-daily or placebo in addition to standard of care. Patients were included if they had positive PCR or diagnostic chest CT, O2 saturation <95% and compatible symptoms. The primary outcome was hospital discharge within 10 days of randomization. Secondary outcomes included mortality and time to clinical events. The trial is registered on the Iran Registry of Clinical Trials under IRCT20200624047908N1.
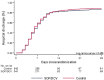
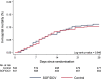
Results: Between July and October 2020, 1083 patients were randomized to either the sofosbuvir/daclatasvir arm (n = 541) or the placebo arm (n = 542). No significant difference was observed in the primary outcome of hospital discharge within 10 days, which was achieved by 415/541 (77%) in the sofosbuvir/daclatasvir arm and 411/542 (76%) in the placebo arm [risk ratio (RR) 1.01, 95% CI 0.95-1.08, P = 0.734]. In-hospital mortality was 60/541 (11%) in the sofosbuvir/daclatasvir arm versus 55/542 (10%) in the placebo arm (RR 1.09, 95% CI 0.77-1.54, P = 0.615). No differences were observed in time to hospital discharge or time to in-hospital mortality.
Conclusions: We observed no significant effect of sofosbuvir/daclatasvir versus placebo on hospital discharge or survival in hospitalized COVID-19 patients.
© The Author(s) 2021. Published by Oxford University Press on behalf of the British Society for Antimicrobial Chemotherapy. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures
References
-
- WHO. Therapeutics and COVID-19: Living Guideline. https://www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2021.1.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

