Long-Term Outcomes With Pharmacological Ovarian Suppression During Chemotherapy in Premenopausal Early Breast Cancer Patients
- PMID: 34850043
- PMCID: PMC8902441
- DOI: 10.1093/jnci/djab213
Long-Term Outcomes With Pharmacological Ovarian Suppression During Chemotherapy in Premenopausal Early Breast Cancer Patients
Abstract
Background: Although use of gonadotropin-releasing hormone agonist (GnRHa) during chemotherapy is an established strategy to protect ovarian function in premenopausal breast cancer patients, no long-term safety data are available, raising some concerns in women with hormone receptor-positive disease. There are controversial data on its fertility preservation potential.
Methods: The Prevention of Menopause Induced by Chemotherapy: a Study in Early Breast Cancer Patients-Gruppo Italiano Mammella 6 (PROMISE-GIM6) trial is a multicenter, randomized, open-label, phase III superiority trial conducted at 16 Italian centers from October 2003 to January 2008. Eligible patients were randomly assigned to (neo)adjuvant chemotherapy alone (control arm) or combined with the GnRHa triptorelin (GnRHa arm). The primary planned endpoint was incidence of chemotherapy-induced premature ovarian insufficiency. Post hoc endpoints were disease-free survival (DFS), overall survival (OS), and post-treatment pregnancies. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated.
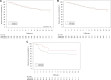
Results: Of 281 randomly assigned patients, 80.4% had hormone receptor-positive breast cancer. Median follow-up was 12.4 years (interquartile range = 11.3-13.2 years). No differences in 12-year DFS (65.7% [95% CI = 57.0% to 73.1%] in the GnRHa arm vs 69.2% [95% CI = 60.3% to 76.5%] in the control arm; HR = 1.16, 95% CI = 0.76 to 1.77) or in 12-year OS (81.2% [95% CI = 73.6% to 86.8%] in the GnRHa arm vs 81.3% [95% CI = 73.1% to 87.2%] in the control arm; HR = 1.17, 95% CI = 0.67 to 2.03) were observed. In patients with hormone receptor-positive disease, the hazard ratio was 1.02 (95% CI = 0.63 to 1.63) for DFS and 1.12 (95% CI = 0.59 to 2.11) for OS. In the GnRHa and control arms, 9 and 4 patients had a posttreatment pregnancy, respectively (HR = 2.14, 95% CI = 0.66 to 6.92).
Conclusions: Final analysis of the PROMISE-GIM6 trial provides reassuring results on the safety of GnRHa use during chemotherapy as a strategy to preserve ovarian function in premenopausal patients with early breast cancer, including those with hormone receptor-positive disease.
© The Author(s) 2021. Published by Oxford University Press. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures

Comment in
-
The Expanded Role of Ovarian Suppression for Young Women's Breast Cancer: An Era of Patient-Tailored Decision Making.J Natl Cancer Inst. 2022 Mar 8;114(3):342-344. doi: 10.1093/jnci/djab214. J Natl Cancer Inst. 2022. PMID: 34850044 Free PMC article. No abstract available.
References
-
- Partridge AH, Hughes ME, Warner ET, et al.Subtype-dependent relationship between young age at diagnosis and breast cancer survival. J Clin Oncol. 2016;34(27):3308–3314. doi:10.1200/JClin Oncol.2015.65.8013. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

