TRF2 promotes dynamic and stepwise looping of POT1 bound telomeric overhang
- PMID: 34850123
- PMCID: PMC8643667
- DOI: 10.1093/nar/gkab1123
TRF2 promotes dynamic and stepwise looping of POT1 bound telomeric overhang
Abstract
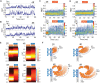
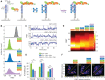
Human telomeres are protected by shelterin proteins, but how telomeres maintain a dynamic structure remains elusive. Here, we report an unexpected activity of POT1 in imparting conformational dynamics of the telomere overhang, even at a monomer level. Strikingly, such POT1-induced overhang dynamics is greatly enhanced when TRF2 engages with the telomere duplex. Interestingly, TRF2, but not TRF2ΔB, recruits POT1-bound overhangs to the telomere ds/ss junction and induces a discrete stepwise movement up and down the axis of telomere duplex. The same steps are observed regardless of the length of the POT1-bound overhang, suggesting a tightly regulated conformational dynamic coordinated by TRF2 and POT1. TPP1 and TIN2 which physically connect POT1 and TRF2 act to generate a smooth movement along the axis of the telomere duplex. Our results suggest a plausible mechanism wherein telomeres maintain a dynamic structure orchestrated by shelterin.
© The Author(s) 2021. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures






Similar articles
-
TRF2-tethered TIN2 can mediate telomere protection by TPP1/POT1.Mol Cell Biol. 2014 Apr;34(7):1349-62. doi: 10.1128/MCB.01052-13. Epub 2014 Jan 27. Mol Cell Biol. 2014. PMID: 24469404 Free PMC article.
-
TIN2 is an architectural protein that facilitates TRF2-mediated trans- and cis-interactions on telomeric DNA.Nucleic Acids Res. 2021 Dec 16;49(22):13000-13018. doi: 10.1093/nar/gkab1142. Nucleic Acids Res. 2021. PMID: 34883513 Free PMC article.
-
In vivo stoichiometry of shelterin components.J Biol Chem. 2010 Jan 8;285(2):1457-67. doi: 10.1074/jbc.M109.038026. Epub 2009 Oct 28. J Biol Chem. 2010. PMID: 19864690 Free PMC article.
-
Shelterin proteins and cancer.Asian Pac J Cancer Prev. 2015;16(8):3085-90. doi: 10.7314/apjcp.2015.16.8.3085. Asian Pac J Cancer Prev. 2015. PMID: 25921101 Review.
-
Shelterin: the protein complex that shapes and safeguards human telomeres.Genes Dev. 2005 Sep 15;19(18):2100-10. doi: 10.1101/gad.1346005. Genes Dev. 2005. PMID: 16166375 Review.
Cited by
-
BG4 antibody can recognize telomeric G-quadruplexes harboring destabilizing base modifications and lesions.Nucleic Acids Res. 2024 Feb 28;52(4):1763-1778. doi: 10.1093/nar/gkad1209. Nucleic Acids Res. 2024. PMID: 38153143 Free PMC article.
-
Human POT1 protects the telomeric ds-ss DNA junction by capping the 5' end of the chromosome.Science. 2023 Aug 18;381(6659):771-778. doi: 10.1126/science.adi2436. Epub 2023 Aug 17. Science. 2023. PMID: 37590346 Free PMC article.
-
Emerging accessibility patterns in long telomeric overhangs.Proc Natl Acad Sci U S A. 2022 Jul 26;119(30):e2202317119. doi: 10.1073/pnas.2202317119. Epub 2022 Jul 18. Proc Natl Acad Sci U S A. 2022. PMID: 35858438 Free PMC article.
-
Protocol for generation and regeneration of PEG-passivated slides for single-molecule measurements.STAR Protoc. 2022 Feb 3;3(1):101152. doi: 10.1016/j.xpro.2022.101152. eCollection 2022 Mar 18. STAR Protoc. 2022. PMID: 35146451 Free PMC article.
-
Mechanistic insights into direct DNA and RNA strand transfer and dynamic protein exchange of SSB and RPA.Nucleic Acids Res. 2025 Jun 20;53(12):gkaf642. doi: 10.1093/nar/gkaf642. Nucleic Acids Res. 2025. PMID: 40598891 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

