Safety and efficacy of dapagliflozin in patients with focal segmental glomerulosclerosis: a prespecified analysis of the dapagliflozin and prevention of adverse outcomes in chronic kidney disease (DAPA-CKD) trial
- PMID: 34850160
- PMCID: PMC9395378
- DOI: 10.1093/ndt/gfab335
Safety and efficacy of dapagliflozin in patients with focal segmental glomerulosclerosis: a prespecified analysis of the dapagliflozin and prevention of adverse outcomes in chronic kidney disease (DAPA-CKD) trial
Abstract
Background: Despite renin-angiotensin-aldosterone system blockade and immunosuppressive treatment, focal segmental glomerulosclerosis (FSGS) often progresses to kidney failure. The objective of this prespecified analysis of the dapagliflozin and prevention of adverse outcomes in chronic kidney disease trial (DAPA-CKD) was to assess efficacy and safety of dapagliflozin in a small subgroup of participants with FSGS confirmed by kidney biopsy.
Methods: In DAPA-CKD, patients with an estimated glomerular filtration rate (eGFR) 25-75 mL/min/1.73 m2 and urinary albumin:creatinine ratio (UACR) 200-5000 mg/g (22.6-565 mg/mol) were randomized to dapagliflozin 10 mg once daily or placebo as an adjunct to standard care and followed for median 2.4 years. The primary composite endpoint was sustained eGFR decline ≥50%, end-stage kidney disease, or kidney or cardiovascular death. The endpoint of interest for this analysis was eGFR slope (acute effects from baseline to Week 2 and chronic effects from Week 2 to end of treatment).
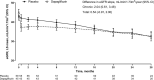
Results: Of 104 participants with biopsy-confirmed FSGS, 45 were randomized to dapagliflozin and 59 to placebo. Mean (standard deviation) age was 54.0 (14.3) years, mean eGFR 41.9 (11.5) mL/min/1.73 m2 and median (interquartile range) UACR 1248 (749-2211) mg/g. The primary outcome occurred in 4 (8.9%) and 7 (11.9%) participants randomized to dapagliflozin and placebo, respectively [hazard ratio 0.62, 95% confidence interval (95% CI) 0.17, 2.17]. Dapagliflozin led to a larger acute reduction (standard error) in eGFR compared with placebo (-4.5, 95% CI -5.9 to -3.1 versus -0.9, -2.1 to 0.4 mL/min/1.73 m2/2 weeks). Thereafter, mean rates of chronic eGFR decline with dapagliflozin and placebo were -1.9 (-3.0, -0.9) and -4.0 (-4.9, -3.0) mL/min/1.73 m2/year, respectively (difference 2.0, 95% CI 0.6 to 3.5, mL/min/1.73 m2/year). Adverse events leading to study drug discontinuation were similar in both groups; there were fewer serious adverse events with dapagliflozin.
Conclusions: Among DAPA-CKD participants with FSGS, dapagliflozin reduced the rate of chronic decline of eGFR compared with placebo, although this difference was not statistically significant.
Keywords: DAPA-CKD; dapagliflozin; eGFR slope; focal segmental glomerulosclerosis.
© The Author(s) 2021. Published by Oxford University Press on behalf of the ERA.
Figures

Comment in
-
Could SGLT2 inhibitors be the next 'game changer' in focal segmental glomerulosclerosis?Nephrol Dial Transplant. 2022 Aug 22;37(9):1593-1594. doi: 10.1093/ndt/gfac139. Nephrol Dial Transplant. 2022. PMID: 35348743 No abstract available.
References
-
- McGrogan A, Franssen CF, de Vries CS.. The incidence of primary glomerulonephritis worldwide: a systematic review of the literature. Nephrol Dial Transplant 2011; 26: 414–430 - PubMed
-
- Barisoni L, Schnaper HW, Kopp JB.. A proposed taxonomy for the podocytopathies: a reassessment of the primary nephrotic diseases. Clin J Am Soc Nephrol 2007; 2: 529–542 - PubMed
-
- D'Agati VD, Kaskel FJ, Falk RJ.. Focal segmental glomerulosclerosis. N Engl J Med 2011; 365: 2398–2411 - PubMed
-
- Rovin BH, Caster DJ, Cattran DCet al. Management and treatment of glomerular diseases (part 2): conclusions from a kidney disease: improving global outcomes (KDIGO) controversies conference. Kidney Int 2019; 95: 281–295 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

