Effect of Electronic Nicotine Delivery Systems on Cigarette Abstinence in Smokers With No Plans to Quit: Exploratory Analysis of a Randomized Placebo-Controlled Trial
- PMID: 34850164
- PMCID: PMC9391685
- DOI: 10.1093/ntr/ntab247
Effect of Electronic Nicotine Delivery Systems on Cigarette Abstinence in Smokers With No Plans to Quit: Exploratory Analysis of a Randomized Placebo-Controlled Trial
Abstract
Introduction: The extent to which use of electronic nicotine delivery systems (ENDS) for smoking reduction leads to cigarette abstinence in smokers with no plans to quit smoking is unclear. This exploratory analysis examined the effects of ENDS delivering different amounts of nicotine on cigarette abstinence up to 24-week follow-up, in comparison to placebo or a behavioral substitute.
Methods: This four-arm parallel-group, randomized, placebo-controlled trial took place at two academic medical centers in the United States (Penn State Hershey and Virginia Commonwealth University). Participants were current adult smokers (N = 520) interested in reducing but not planning to quit. They received brief advice and were randomized to one of four 24-week conditions, receiving either an eGo-style ENDS paired with 0, 8, or 36 mg/ml nicotine liquid (double-blind) or a cigarette-shaped tube, as a cigarette substitute (CS). Self-reported daily cigarette consumption and exhaled carbon monoxide (CO) were measured at all study visits. Outcomes included intent-to-treat, self-reported 7-day cigarette abstinence, biochemically confirmed by exhaled CO at 24 weeks after randomization.
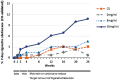
Results: At 24 weeks, significantly more participants in the 36 mg/ml condition (14/130, 10.8%) than in the 0 mg/ml condition (1/130, 0.8%) and the CS condition (4/130, 3.1%) were abstinent (relative risk = 14 [95% CI = 1.9-104.9] and 3.5 [95% CI = 1.2-10.4], respectively). The abstinence rate in the 8 mg/ml condition was 4.6% (6/130).
Conclusions: When smokers seeking to reduce smoking tried ENDS, few quit smoking in the short term. However, if smokers continued to use an ENDS with cigarette-like nicotine delivery, a greater proportion completely switched to ENDS, as compared with placebo or a cigarette substitute.
Implications: The extent to which use of electronic nicotine delivery systems (ENDS) for smoking reduction leads to cigarette abstinence in smokers with no plans to quit smoking was unclear. This randomized trial found that ENDS with nicotine delivery approaching that of a cigarette are more effective in helping ambivalent smokers to quit cigarette smoking.
© The Author(s) 2021. Published by Oxford University Press on behalf of the Society for Research on Nicotine and Tobacco. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures

Comment in
-
E-Cigarettes, Harm Reduction, and Smoking Cessation: Where Are We Now?Nicotine Tob Res. 2022 Jun 15;24(7):943-944. doi: 10.1093/ntr/ntac105. Nicotine Tob Res. 2022. PMID: 35427419 No abstract available.
References
-
- National Academies of Science Engineering and Medicine. Public Health Consequences of E-cigarettes. Washington, DC.The National Academies Press; 2018. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

