Efficacy and safety of larotrectinib in TRK fusion-positive primary central nervous system tumors
- PMID: 34850167
- PMCID: PMC9159442
- DOI: 10.1093/neuonc/noab274
Efficacy and safety of larotrectinib in TRK fusion-positive primary central nervous system tumors
Abstract
Background: Larotrectinib is a first-in-class, highly selective tropomyosin receptor kinase (TRK) inhibitor approved to treat adult and pediatric patients with TRK fusion-positive cancer. The aim of this study was to evaluate the efficacy and safety of larotrectinib in patients with TRK fusion-positive primary central nervous system (CNS) tumors.
Methods: Patients with TRK fusion-positive primary CNS tumors from two clinical trials (NCT02637687, NCT02576431) were identified. The primary endpoint was investigator-assessed objective response rate (ORR).
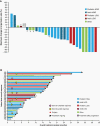
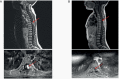
Results: As of July 2020, 33 patients with TRK fusion-positive CNS tumors were identified (median age: 8.9 years; range: 1.3-79.0). The most common histologies were high-grade glioma (HGG; n = 19) and low-grade glioma (LGG; n = 8). ORR was 30% (95% confidence interval [CI]: 16-49) for all patients. The 24-week disease control rate was 73% (95% CI: 54-87). Twenty-three of 28 patients (82%) with measurable disease had tumor shrinkage. The 12-month rates for duration of response, progression-free survival, and overall survival were 75% (95% CI: 45-100), 56% (95% CI: 38-74), and 85% (95% CI: 71-99), respectively. Median time to response was 1.9 months (range 1.0-3.8 months). Duration of treatment ranged from 1.2-31.3+ months. Treatment-related adverse events were reported for 20 patients, with grade 3-4 in 3 patients. No new safety signals were identified.
Conclusions: In patients with TRK fusion-positive CNS tumors, larotrectinib demonstrated rapid and durable responses, high disease control rate, and a favorable safety profile.
Keywords: NTRK gene fusions; TRK fusion; larotrectinib; primary CNS tumors.
© The Author(s) 2021. Published by Oxford University Press on behalf of the Society for Neuro-Oncology.
Figures
Comment in
-
TRK inhibition for pediatric and adult central nervous system tumors: Early promise and future questions.Neuro Oncol. 2022 Jun 1;24(6):1008-1009. doi: 10.1093/neuonc/noac048. Neuro Oncol. 2022. PMID: 35178549 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

