Effects of canagliflozin versus finerenone on cardiorenal outcomes: exploratory post hoc analyses from FIDELIO-DKD compared to reported CREDENCE results
- PMID: 34850173
- PMCID: PMC9217637
- DOI: 10.1093/ndt/gfab336
Effects of canagliflozin versus finerenone on cardiorenal outcomes: exploratory post hoc analyses from FIDELIO-DKD compared to reported CREDENCE results
Abstract
Background: The nonsteroidal mineralocorticoid receptor antagonist finerenone and the sodium-glucose cotransporter-2 inhibitor (SGLT-2i) canagliflozin reduce cardiorenal risk in albuminuric patients with chronic kidney disease (CKD) and type 2 diabetes (T2D). At first glance, the results of Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease (FIDELIO-DKD) (ClinicalTrials.gov, NCT02540993) and Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) appear disparate. In FIDELIO-DKD, the primary endpoint had an 18% [95% confidence interval (CI) 7-27] relative risk reduction; in CREDENCE, the primary endpoint had a 30% (95% CI 18-41) relative risk reduction. Unlike CREDENCE, the FIDELIO-DKD trial included patients with high albuminuria but excluded patients with symptomatic heart failure with reduced ejection fraction. The primary endpoint in the FIDELIO-DKD trial was kidney specific and included a sustained decline in the estimated glomerular filtration rate (eGFR) of ≥40% from baseline. In contrast, the primary endpoint in the CREDENCE trial included a sustained decline in eGFR of ≥57% from baseline and cardiovascular (CV) death. This post hoc exploratory analysis investigated how differences in trial design-inclusion/exclusion criteria and definition of primary outcomes-influenced observed treatment effects.
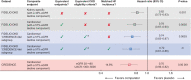
Methods: Patients from FIDELIO-DKD who met the CKD inclusion criteria of the CREDENCE study (urine albumin: creatinine ratio >300-5000 mg/g and an eGFR of 30-<90 mL/min/1.73 m2 at screening) were included in this analysis. The primary endpoint was a cardiorenal composite (CV death, kidney failure, eGFR decrease of ≥57% sustained for ≥4 weeks or renal death). Patients with symptomatic heart failure with reduced ejection fraction were excluded from FIDELIO-DKD. Therefore, in a sensitivity analysis, we further adjusted for the baseline prevalence of heart failure.
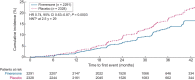
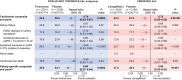
Results: Of 4619/5674 (81.4%) patients who met the subgroup inclusion criteria, 49.6% were treated with finerenone and 50.4% received placebo. The rate of the cardiorenal composite endpoint was 43.9/1000 patient-years with finerenone compared with 59.5/1000 patient-years with placebo. The relative risk was significantly reduced by 26% with finerenone versus placebo [hazard ratio (HR) 0.74 (95% CI 0.63-0.87)]. In CREDENCE, the rate of the cardiorenal composite endpoint was 43.2/1000 patient-years with canagliflozin compared with 61.2/1000 patient-years with placebo; a 30% risk reduction was observed with canagliflozin [HR 0.70 (95% CI 0.59-0.82)].
Conclusions: This analysis highlights the pitfalls of direct comparisons between trials. When key differences in trial design are considered, FIDELIO-DKD and CREDENCE demonstrate cardiorenal benefits of a similar magnitude.
Keywords: CREDENCE; FIDELIO-DKD; canagliflozin; cardiorenal; finerenone.
© The Author(s) 2021. Published by Oxford University Press on behalf of the ERA.
Figures

Comment in
-
SGLT2 inhibitors and finerenone: one or the other or both?Nephrol Dial Transplant. 2022 Jun 23;37(7):1209-1211. doi: 10.1093/ndt/gfac046. Nephrol Dial Transplant. 2022. PMID: 35212745 No abstract available.
Similar articles
-
Finerenone in patients with chronic kidney disease and type 2 diabetes with and without heart failure: a prespecified subgroup analysis of the FIDELIO-DKD trial.Eur J Heart Fail. 2022 Jun;24(6):996-1005. doi: 10.1002/ejhf.2469. Epub 2022 May 19. Eur J Heart Fail. 2022. PMID: 35239204 Free PMC article. Clinical Trial.
-
Cardiorenal Outcomes with Finerenone in Asian Patients with Chronic Kidney Disease and Type 2 Diabetes: A FIDELIO-DKD post hoc Analysis.Am J Nephrol. 2023;54(9-10):370-378. doi: 10.1159/000532102. Epub 2023 Sep 14. Am J Nephrol. 2023. PMID: 37708857 Clinical Trial.
-
Finerenone and Heart Failure Outcomes by Kidney Function/Albuminuria in Chronic Kidney Disease and Diabetes.JACC Heart Fail. 2022 Nov;10(11):860-870. doi: 10.1016/j.jchf.2022.07.013. Epub 2022 Oct 12. JACC Heart Fail. 2022. PMID: 36328655 Clinical Trial.
-
Class effects of SGLT2 inhibitors on cardiorenal outcomes.Cardiovasc Diabetol. 2019 Aug 5;18(1):99. doi: 10.1186/s12933-019-0903-4. Cardiovasc Diabetol. 2019. PMID: 31382965 Free PMC article. Review.
-
Renal and Cardiovascular Effects of Sodium Glucose Co-Transporter 2 Inhibitors in Patients with Type 2 Diabetes and Chronic Kidney Disease: Perspectives on the Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation Trial Results.Am J Nephrol. 2020;51(4):276-288. doi: 10.1159/000506533. Epub 2020 Mar 13. Am J Nephrol. 2020. PMID: 32172239 Review.
Cited by
-
Renal Protection of Mineralocorticoid Receptor Antagonist, Finerenone, in Diabetic Kidney Disease.Endocrinol Metab (Seoul). 2023 Feb;38(1):43-55. doi: 10.3803/EnM.2022.1629. Epub 2023 Feb 27. Endocrinol Metab (Seoul). 2023. PMID: 36891650 Free PMC article. Review.
-
The role of a novel mineralocorticoid receptor antagonist, finerenone, in chronic kidney disease: mechanisms and clinical advances.Clin Exp Nephrol. 2024 Feb;28(2):125-135. doi: 10.1007/s10157-023-02413-2. Epub 2023 Oct 17. Clin Exp Nephrol. 2024. PMID: 37847437 Review.
-
Comparative Efficacy of Finerenone versus Canagliflozin in Patients with Chronic Kidney Disease and Type 2 Diabetes: A Matching-Adjusted Indirect Comparison.J Mark Access Health Policy. 2024 Jul 25;12(3):169-180. doi: 10.3390/jmahp12030014. eCollection 2024 Sep. J Mark Access Health Policy. 2024. PMID: 39193541 Free PMC article.
-
A new perspective on proteinuria and drug therapy for diabetic kidney disease.Front Pharmacol. 2024 Jul 31;15:1349022. doi: 10.3389/fphar.2024.1349022. eCollection 2024. Front Pharmacol. 2024. PMID: 39144629 Free PMC article. Review.
-
The evolution of "pillars of therapy" to reduce heart failure risk and slow diabetic kidney disease progression.Am Heart J Plus. 2022 Aug 5;19:100187. doi: 10.1016/j.ahjo.2022.100187. eCollection 2022 Jul. Am Heart J Plus. 2022. PMID: 38558865 Free PMC article.
References
-
- Bakris GL, Agarwal R, Anker SDet al. . Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med 2020; 383: 2219–2229 - PubMed
-
- Lewis EJ, Hunsicker LG, Clarke WRet al. . Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001; 345: 851–860 - PubMed
-
- Brenner BM, Cooper ME, de Zeeuw Det al. . Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345: 861–869 - PubMed
-
- Perkovic V, Jardine MJ, Neal Bet al. . Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019; 380: 2295–2306 - PubMed
-
- Heerspink HJL, Stefánsson BV, Correa-Rotter Ret al. . Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020; 383: 1436–1446 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

