Immunotherapy of HBV-related advanced hepatocellular carcinoma with short-term HBV-specific TCR expressed T cells: results of dose escalation, phase I trial
- PMID: 34850325
- PMCID: PMC8651587
- DOI: 10.1007/s12072-021-10250-2
Immunotherapy of HBV-related advanced hepatocellular carcinoma with short-term HBV-specific TCR expressed T cells: results of dose escalation, phase I trial
Abstract
Background & aims: Immunotherapy with hepatitis B virus (HBV)-specific TCR redirected T (HBV-TCR-T) cells in HBV-related hepatocellular carcinoma (HBV-HCC) patients after liver transplantation was reported to be safe and had potential therapeutic efficacy. We aim to investigate the safety of HBV-TCR-T-cell immunotherapy in advanced HBV-HCC patients who had not met the criteria for liver transplantation.
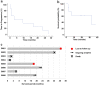
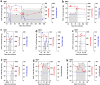
Methods: We enrolled eight patients with advanced HBV-HCC and adoptively transferred short-lived autologous T cells expressing HBV-specific TCR to perform an open-label, phase 1 dose-escalation study (NCT03899415). The primary endpoint was to evaluate the safety of HBV-TCR-T-cell therapy according to National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.03) during the dose-escalation process. The secondary endpoint was to assess the efficacy of HBV-TCR-T-cell therapy by evaluating the anti-tumor responses using RECIST criteria (version 1.1) and the overall survival.
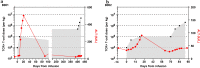
Results: Adverse events were observed in two participants among the 8 patients enrolled. Only one patient experienced a Grade 3 liver-related adverse event after receiving a dose of 1 × 105 HBV-TCR-T cells/kg, then normalized without interventions with immunosuppressive agents. Among the patients, one achieved a partial response lasting for 27.7 months. Importantly, most of the patients exhibited a reduction or stabilization of circulating HBsAg and HBV DNA levels after HBV-TCR-T-cell infusion, indicating the on-target effects.
Conclusions: The adoptive transfer of HBV-TCR-T cells into advanced HBV-HCC patients were generally safe and well-tolerated. Observations of clinical efficacy support the continued development and eventual application of this treatment strategy in patients with advanced HBV-related HCC.
Clinical trials registration: This study was registered at ClinicalTrials.gov (NCT03899415).
Keywords: Chronic hepatitis B; Clinical trial; HBV; HBV-TCR-T cells; HCC; Immunotherapy; Overall survival; Phase 1; Safety; Time-to-progression.
© 2021. The Author(s).
Conflict of interest statement
Antonio Bertoletti is a co-founder of Lion TCR Pte. Ltd. a biotech company developing T cell receptors for treatment of virus-related cancers and chronic viral diseases. Anthony T. Tan is a scientific consultant for Lion TCR Pte. Ltd. Regina Wanju Wong, Wai Lu-En, Sarene Koh and Tingting Wang are employees of Lion TCR Pte. Ltd. Fanping Meng, Jinfang Zhao, Wei Hu, Si-Yu Wang, Jiehua Jin, Juan Wu, Yuanyuan Li, Lei Shi, Jun-Liang Fu, Shuangjie Yu, Yingjuan Shen, Limin Liu, Junqing Luan, Ming Shi, Yunbo Xie, Chun-Bao Zhou, Ji-Yuan Zhang, Fu-Sheng Wang have no conflicts of interest to disclose.
Figures


References
-
- Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. - DOI - PubMed
-
- Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–1173. doi: 10.1016/S0140-6736(18)30207-1. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

