Recent Advances in Heterocyclic Nanographenes and Other Polycyclic Heteroaromatic Compounds
- PMID: 34850633
- PMCID: PMC8759089
- DOI: 10.1021/acs.chemrev.1c00449
Recent Advances in Heterocyclic Nanographenes and Other Polycyclic Heteroaromatic Compounds
Abstract
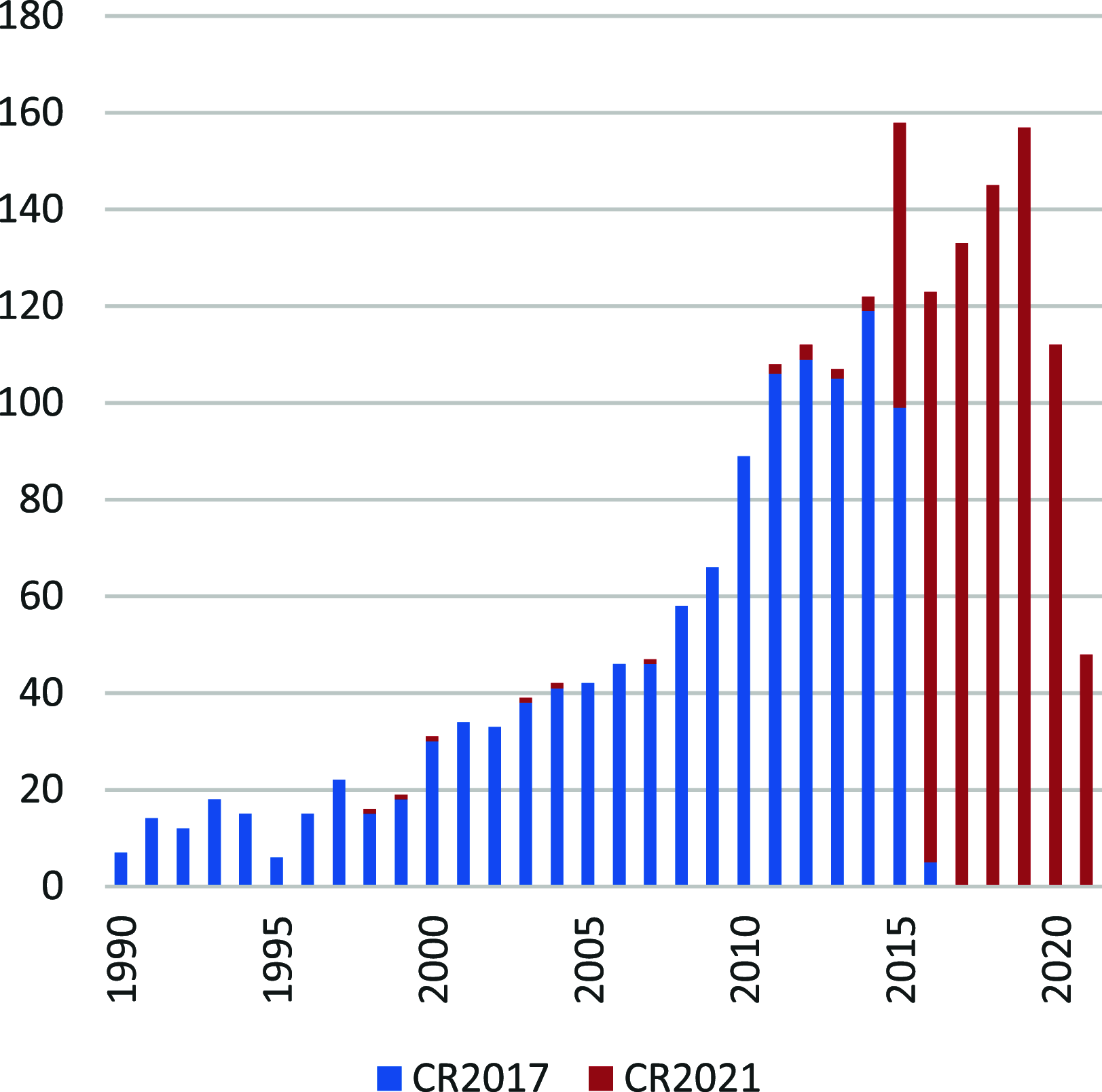
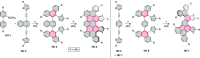
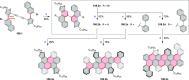
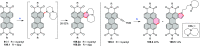
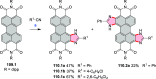
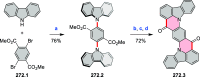
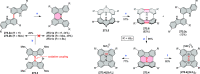
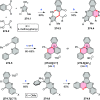
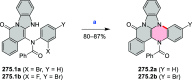
This review surveys recent progress in the chemistry of polycyclic heteroaromatic molecules with a focus on structural diversity and synthetic methodology. The article covers literature published during the period of 2016-2020, providing an update to our first review of this topic (Chem. Rev. 2017, 117 (4), 3479-3716).
Conflict of interest statement
The authors declare no competing financial interest.
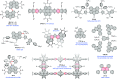
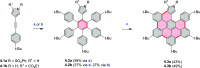
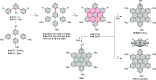
Figures



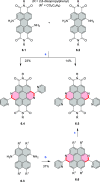

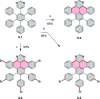
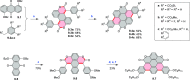
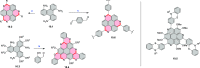
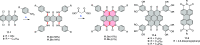
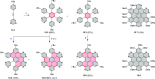

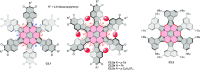
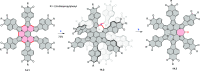
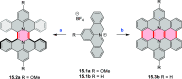
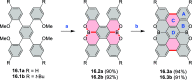
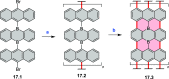

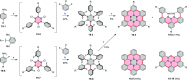
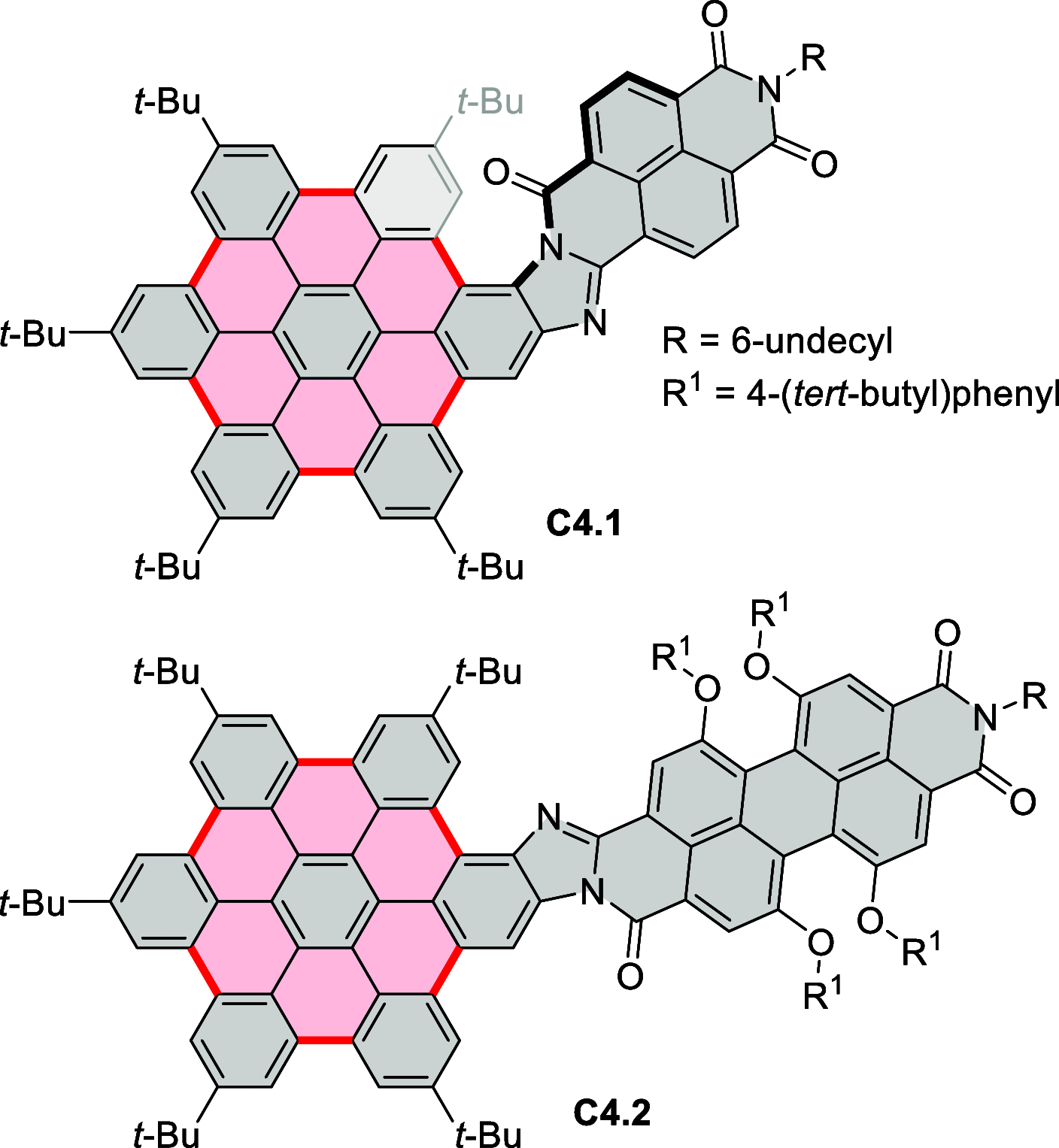
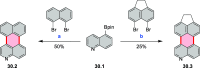
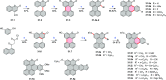
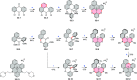
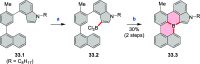
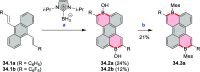
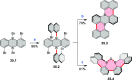
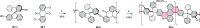

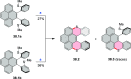
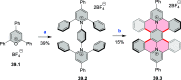
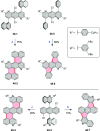

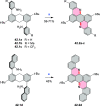

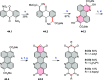




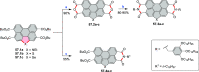
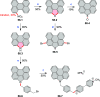
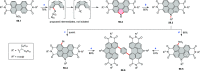


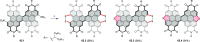
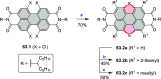

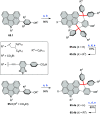
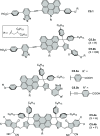
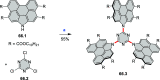
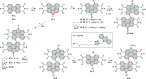
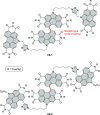
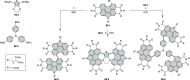
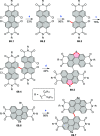



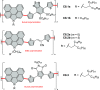
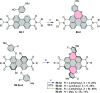
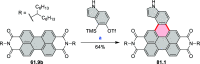
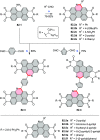
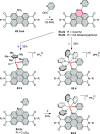
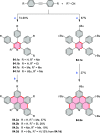
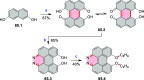
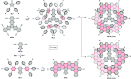
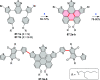
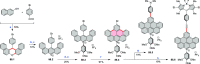

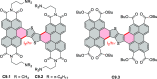


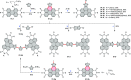
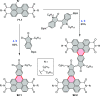
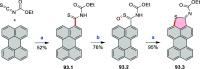


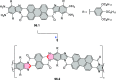
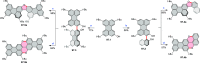
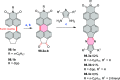
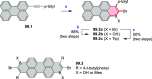

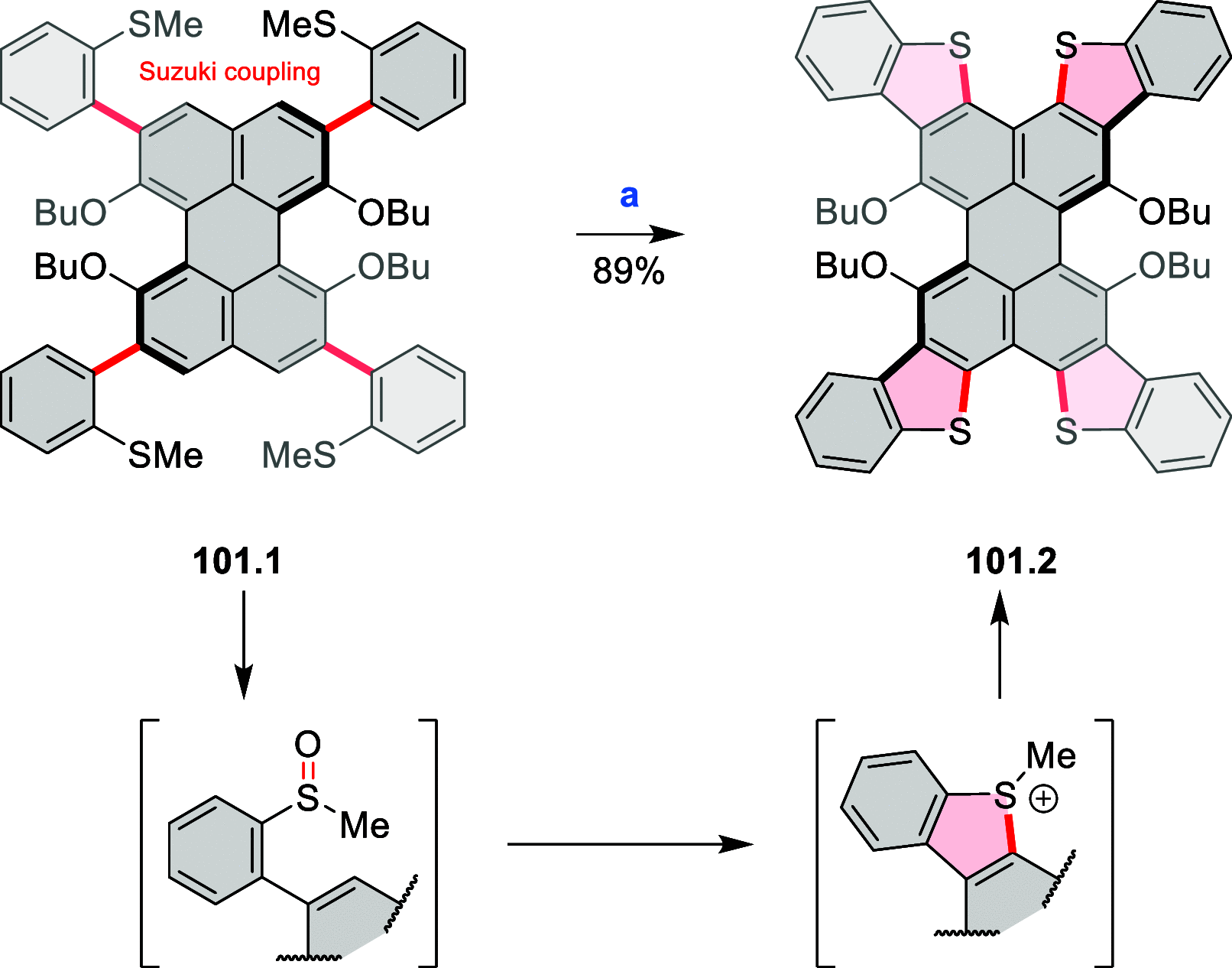
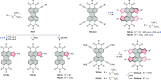
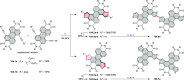
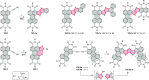
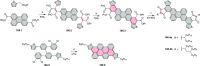


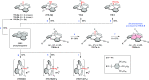
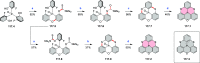
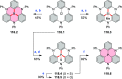
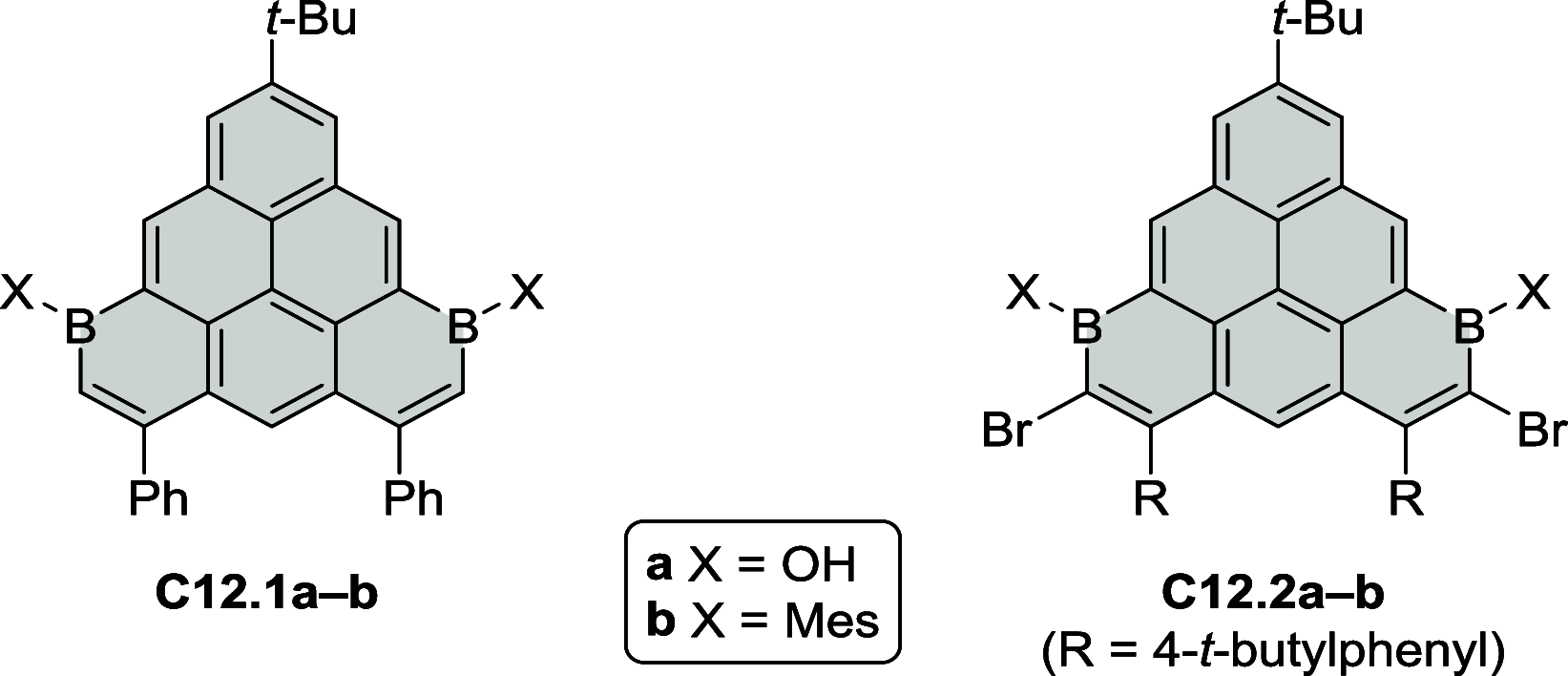


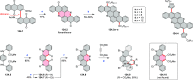
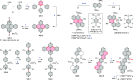


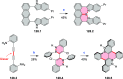
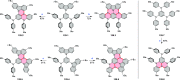
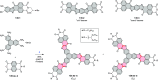
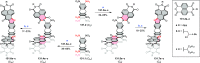

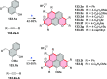
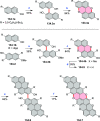
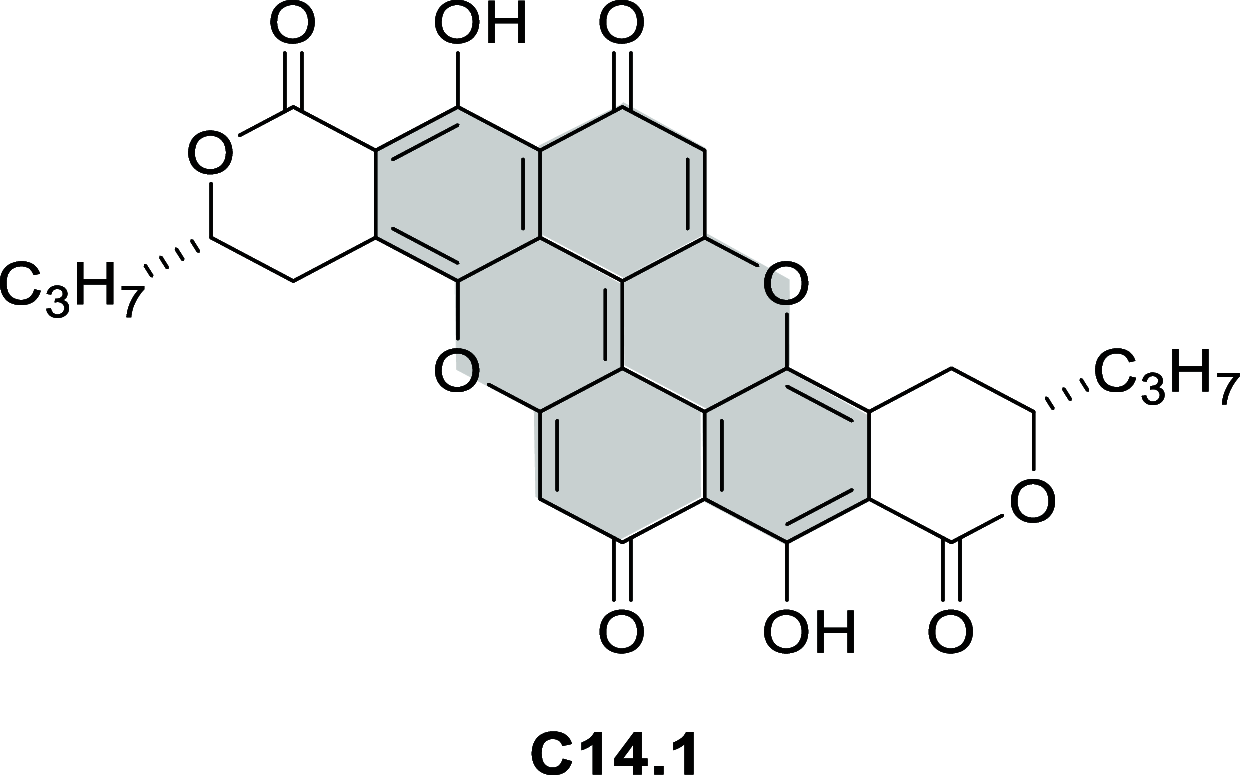


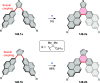
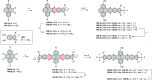
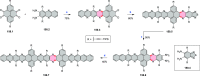
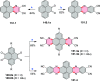

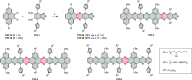
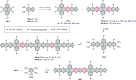
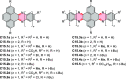

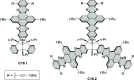




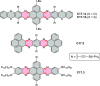
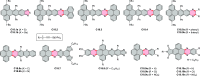
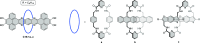
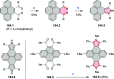

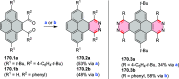
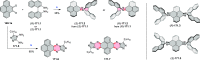
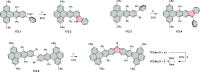
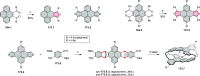


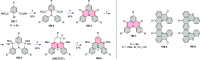

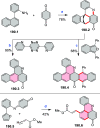
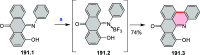
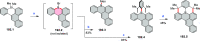



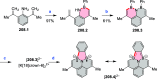
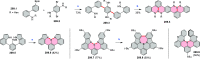

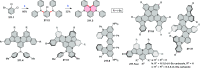
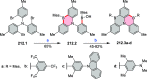

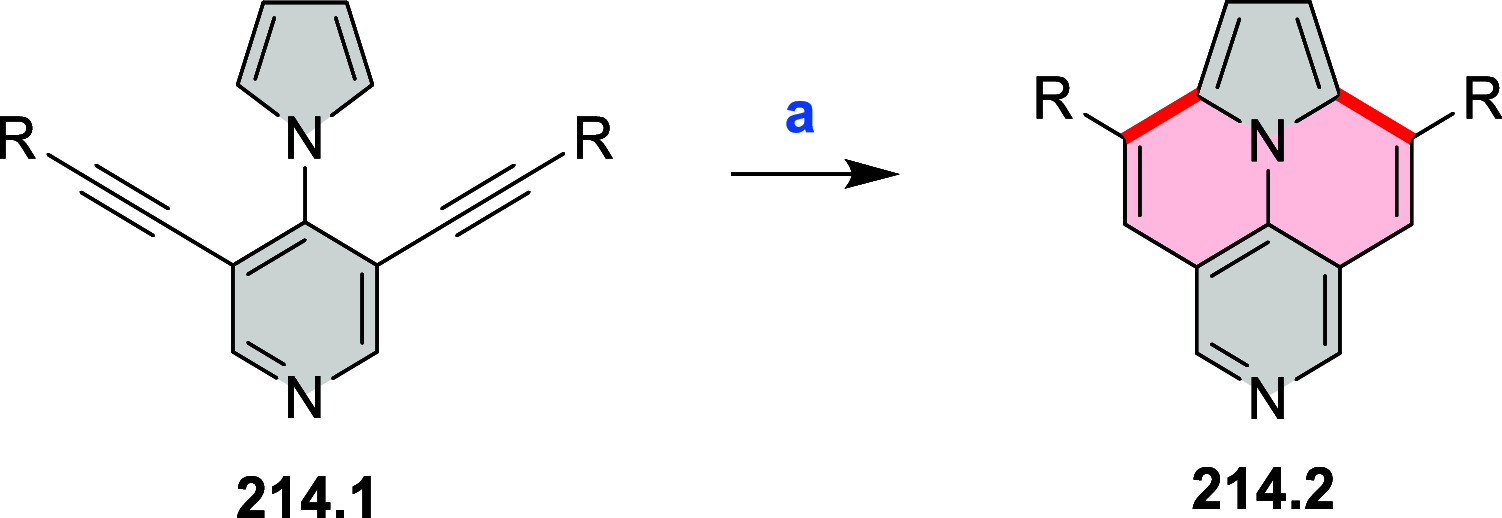
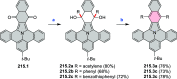
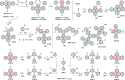
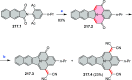




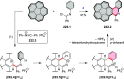
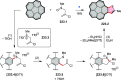
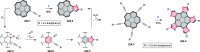

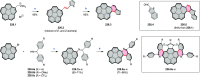
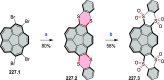
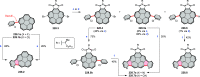
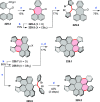
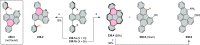
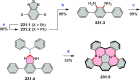


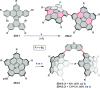
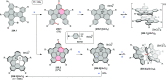

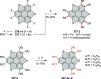
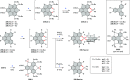
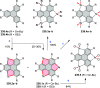

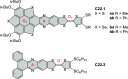

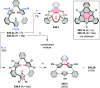
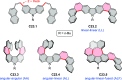


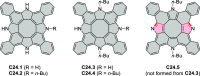
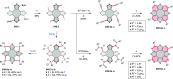
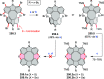
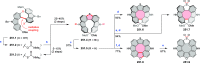

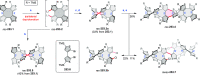
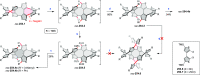
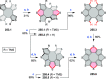

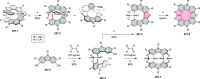
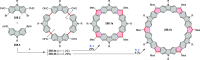

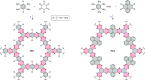
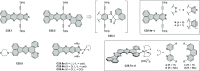
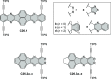



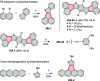
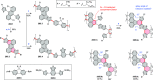


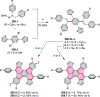
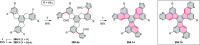
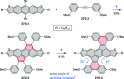

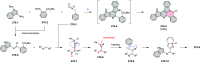
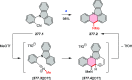
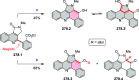

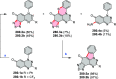
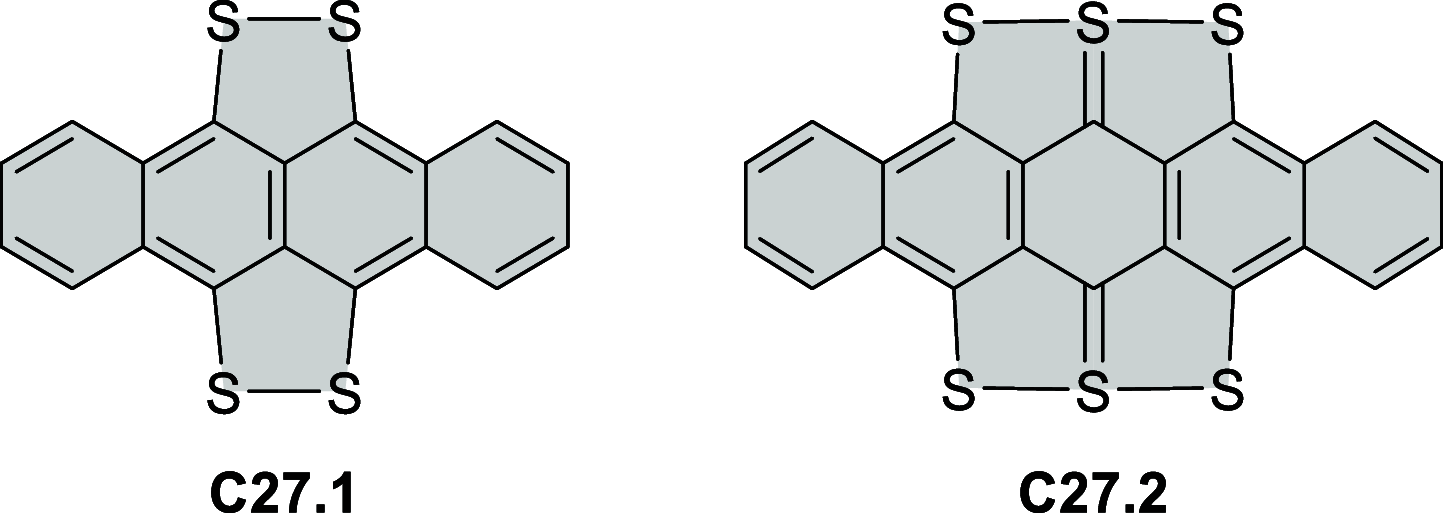


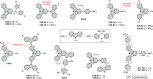

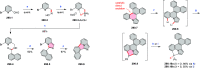
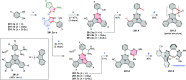
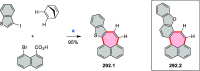












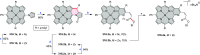
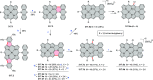


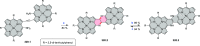
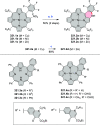


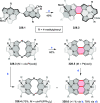
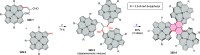
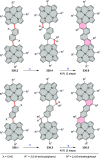
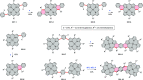








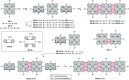



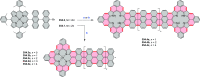

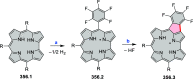
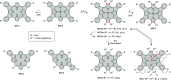




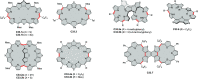
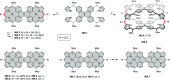
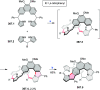
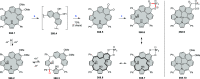
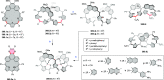

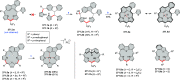

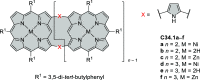
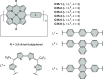

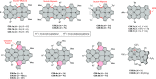
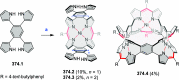
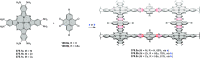
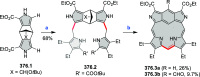


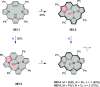

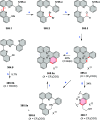

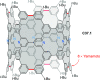


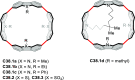
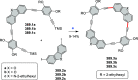
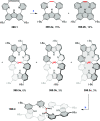



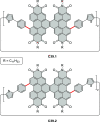

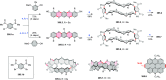
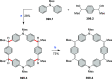

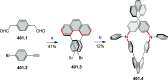
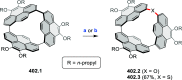
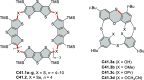
References
-
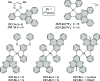
- Ammon F.; Sauer S. T.; Lippert R.; Lungerich D.; Reger D.; Hampel F.; Jux N. Unexpected Formation of [5]Helicenes from Hexaarylbenzenes Containing Pyrrole Moieties. Org. Chem. Front. 2017, 4, 861–870. 10.1039/C7QO00112F. - DOI
-
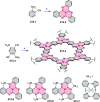
- Greßies S.; Ito M.; Sakai M.; Osaki H.; Kim J. H.; Gensch T.; Daniliuc C.; Ando N.; Yamaguchi S.; Glorius F. Twofold C-H Activation Enables Synthesis of a Diazacoronene-Type Fluorophore with Near Infrared Emission Through Isosteric Replacement. Chem. - Eur. J. 2021, 27, 2753–2759. 10.1002/chem.202004080. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

