MIF but not MIF-2 recruits inflammatory macrophages in an experimental polymicrobial sepsis model
- PMID: 34850744
- PMCID: PMC8631602
- DOI: 10.1172/JCI127171
MIF but not MIF-2 recruits inflammatory macrophages in an experimental polymicrobial sepsis model
Abstract
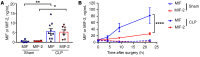
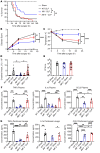
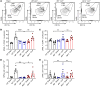
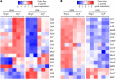
Excessive inflammation drives the progression from sepsis to septic shock. Macrophage migration inhibitory factor (MIF) is of interest because MIF promoter polymorphisms predict mortality in different infections, and anti-MIF antibody improves survival in experimental models when administered 8 hours after infectious insult. The recent description of a second MIF superfamily member, D-dopachrome tautomerase (D-DT/MIF-2), prompted closer investigation of MIF-dependent responses. We subjected Mif-/- and Mif-2-/- mice to polymicrobial sepsis and observed a survival benefit with Mif but not Mif-2 deficiency. Survival was associated with reduced numbers of small peritoneal macrophages (SPMs) that, in contrast to large peritoneal macrophages (LPMs), were recruited into the peritoneal cavity. LPMs produced higher quantities of MIF than SPMs, but SPMs expressed higher levels of inflammatory cytokines and the MIF receptors CD74 and CXCR2. Adoptive transfer of WT SPMs into Mif-/- hosts reduced the protective effect of Mif deficiency in polymicrobial sepsis. Notably, MIF-2 lacks the pseudo-(E)LR motif present in MIF that mediates CXCR2 engagement and SPM migration, supporting a specific role for MIF in the recruitment and accumulation of inflammatory SPMs.
Keywords: Cytokines; Immunology; Inflammation; Macrophages; Mouse models.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

